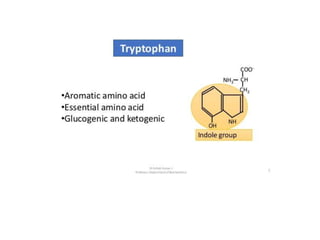
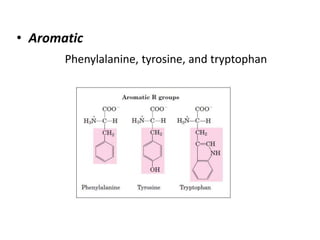
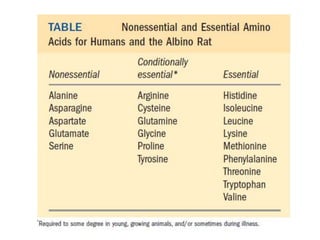
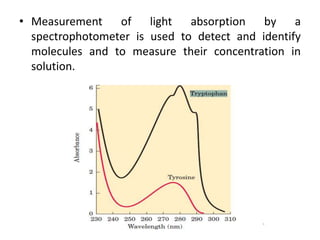
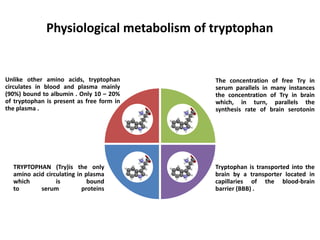
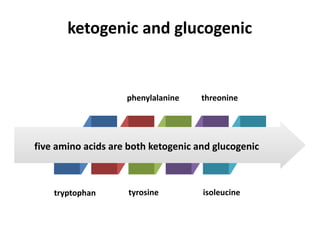
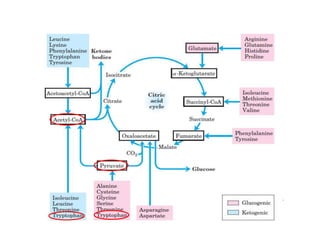
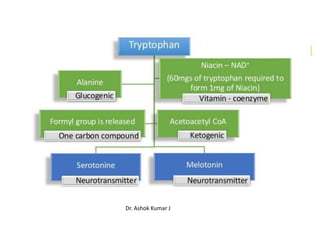
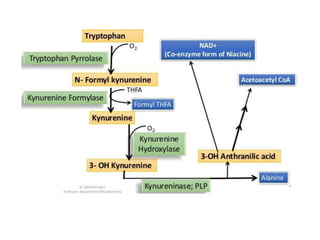
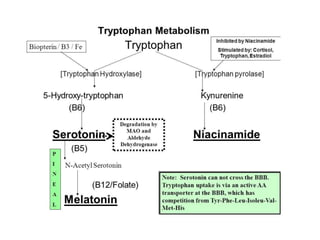
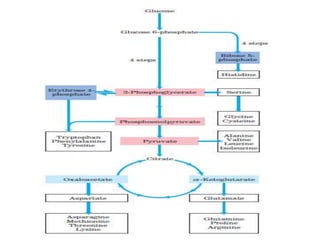
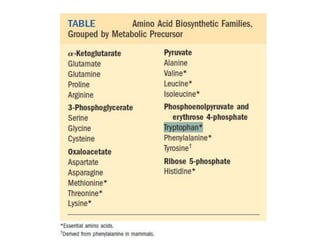
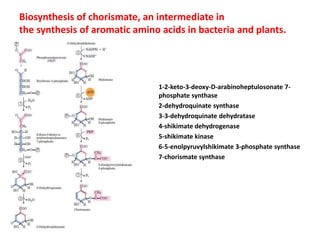
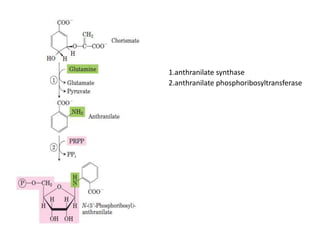
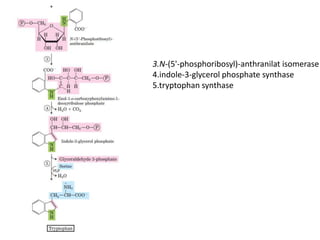
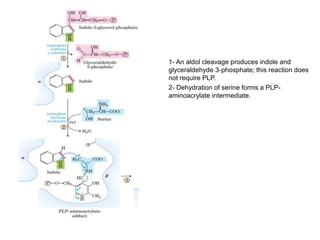
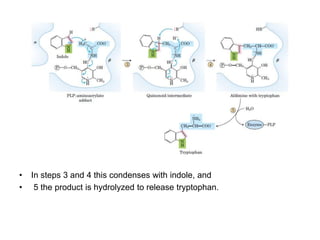
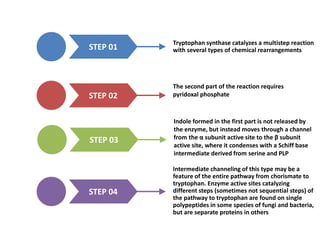
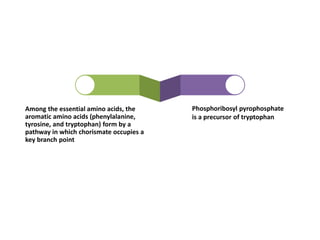
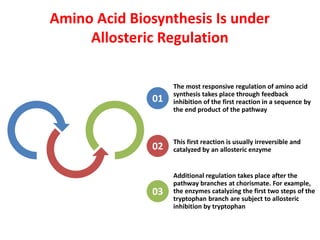
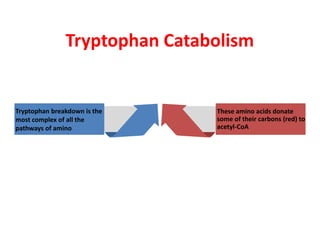
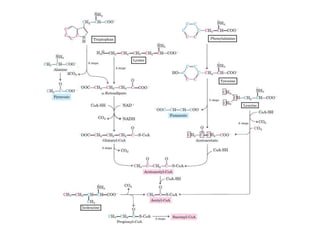
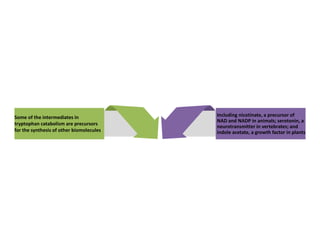
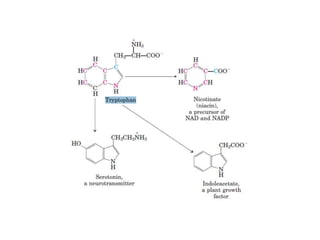
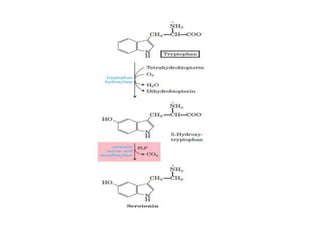
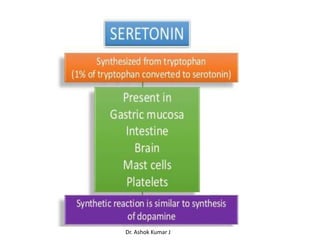
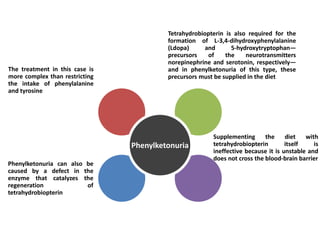
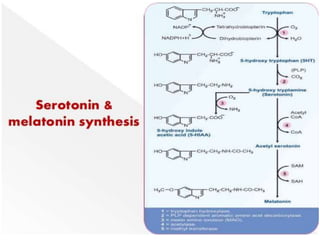
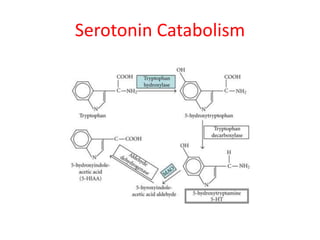
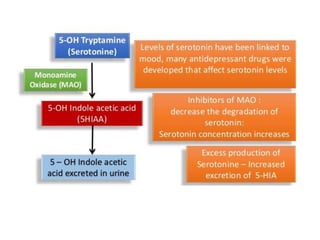
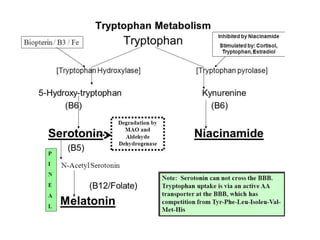
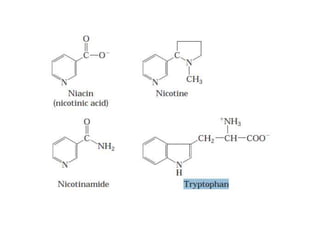
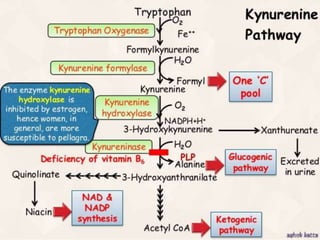
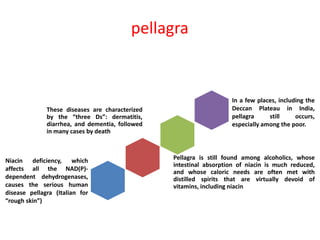
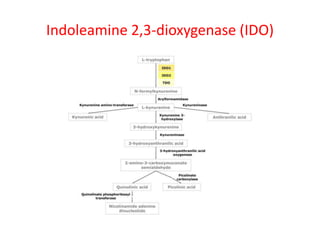
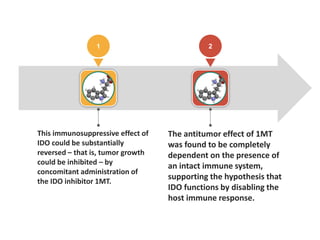
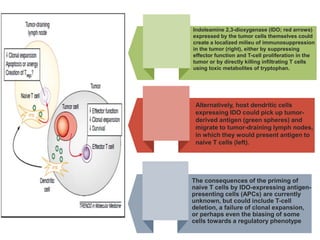
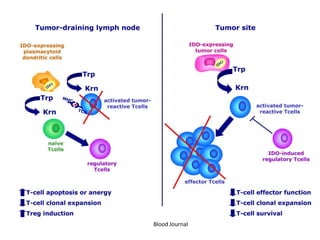
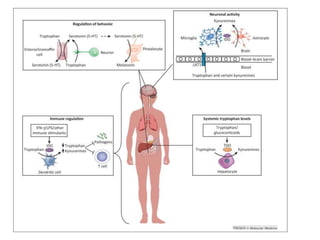
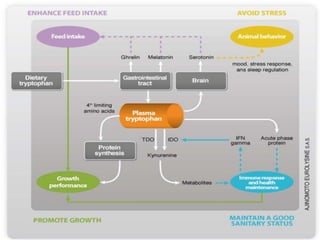
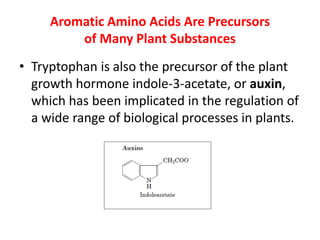
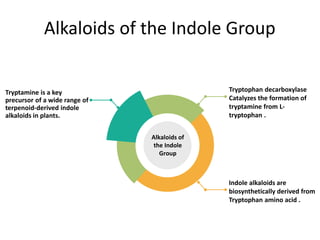
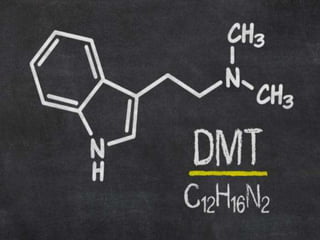
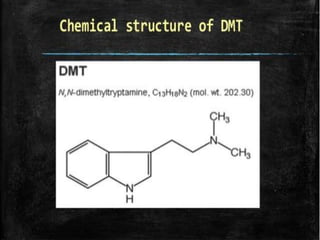
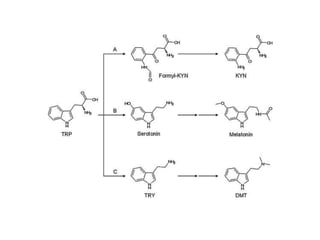
Tryptophan is an essential amino acid that is important for many functions in the body. It is used to produce serotonin, an important neurotransmitter, and niacin, a B vitamin. Tryptophan helps regulate mood and promotes healthy sleep. It is obtained through the diet from protein sources and plays critical roles in various physiological processes as well as the synthesis of important compounds like serotonin and auxins in plants.