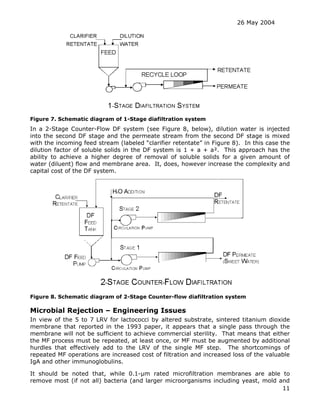
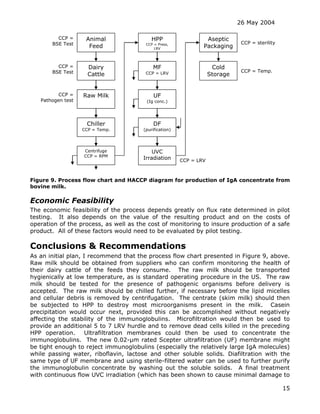
The document discusses the feasibility of using a Scepter composite metallic membrane ultrafiltration system to sterilize an IgA-enriched liquid whey supplement without using thermal sterilization. The Scepter membrane consists of a thin layer of sintered titanium dioxide particles applied to the inner surface of porous stainless steel tubes. The document considers whether the 0.1-micron rated Scepter membrane would be suitable to produce a sterile product and discusses various regulatory, scientific and practical issues that would need to be addressed in developing such a sterilization process.