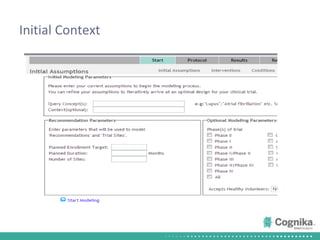
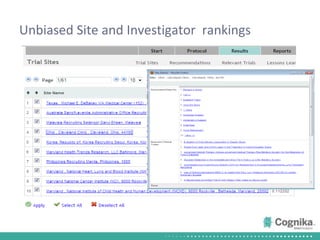
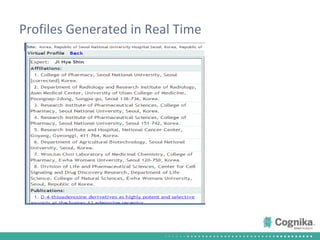
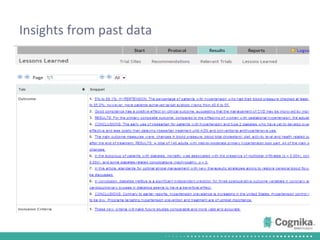
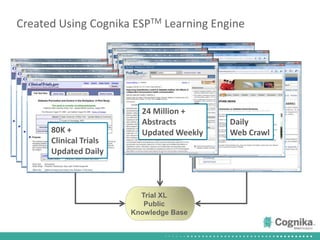
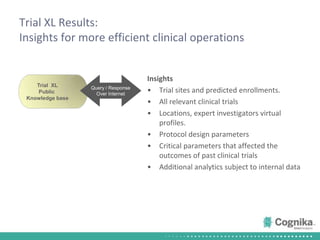
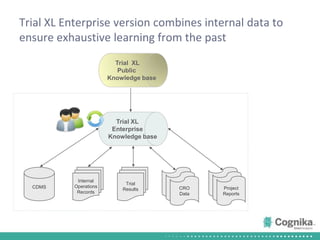
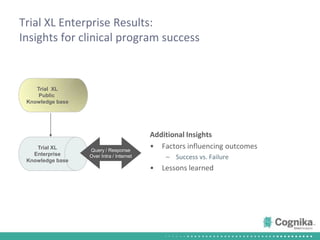
Cognika's Trial XL uses machine learning to analyze clinical trial data and provide insights to improve trial operations. It profiles investigators and sites, evaluates criteria that impact recruitment and outcomes, and ranks investigators. Case studies showed it identified criteria impacting recruitment, better accrual estimates, and new investigators, helping reduce delays. Trial XL leverages all past learning, applies analytics to extract insights, and accelerates decision making for clinical trials.