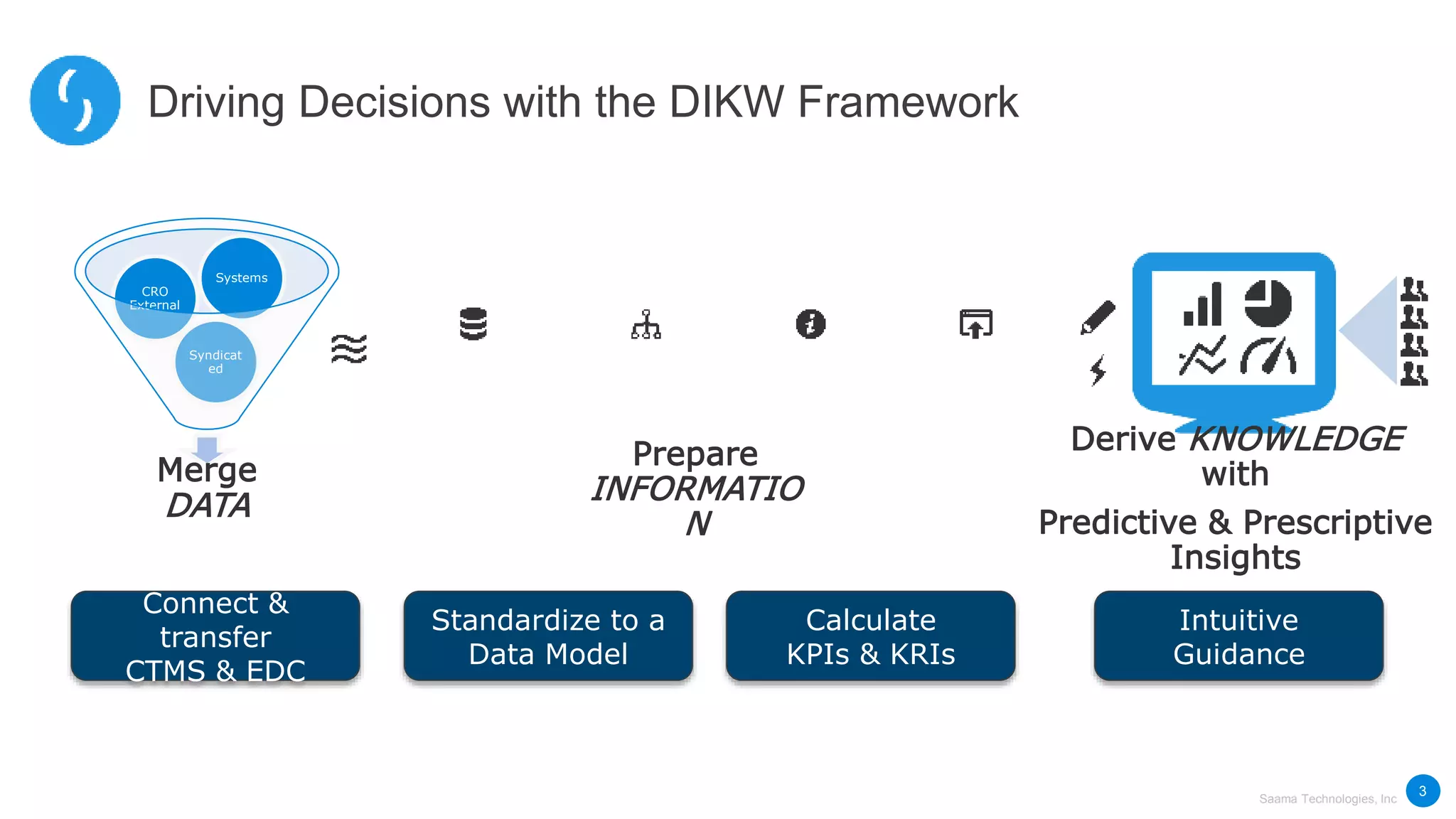
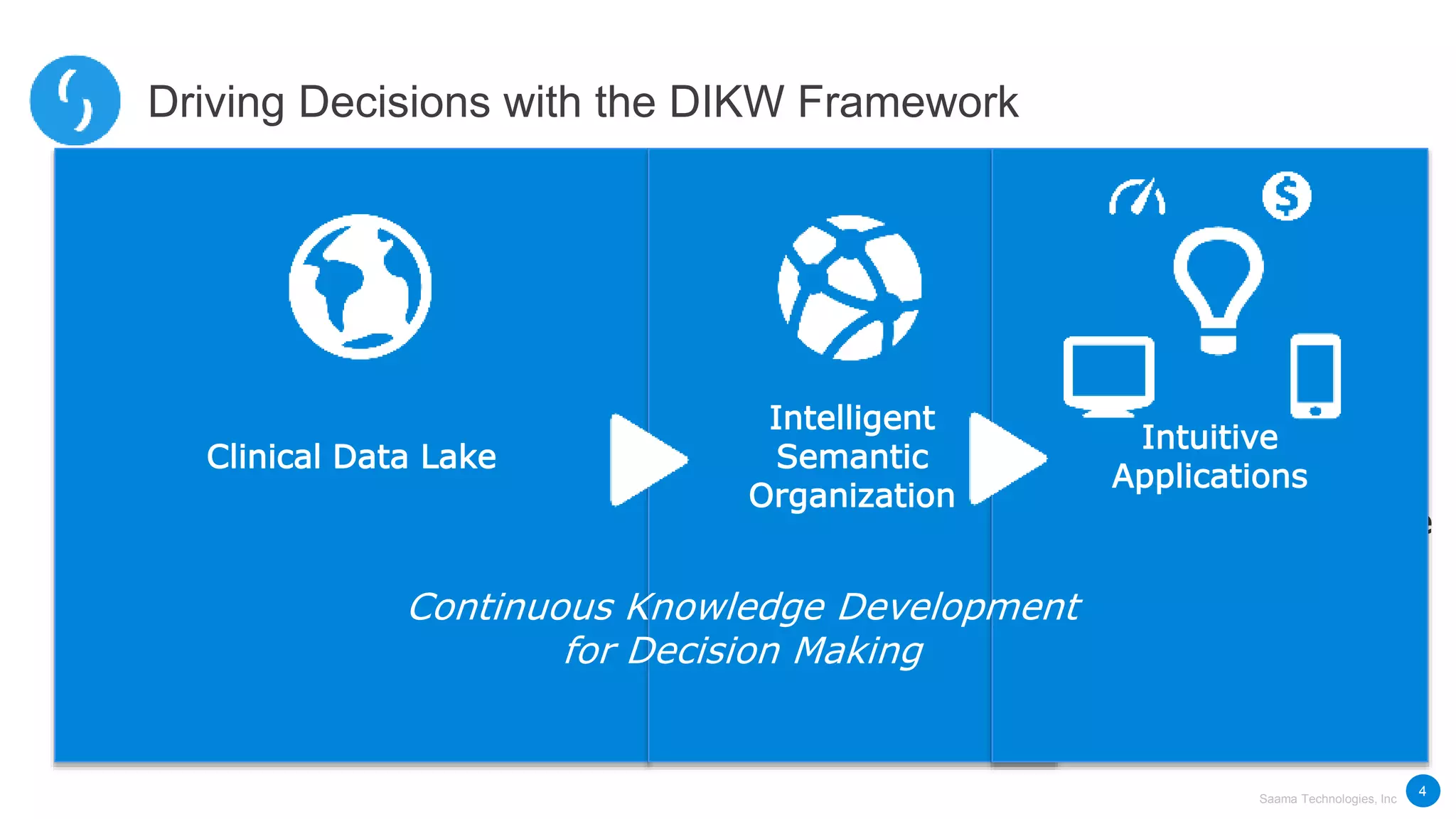
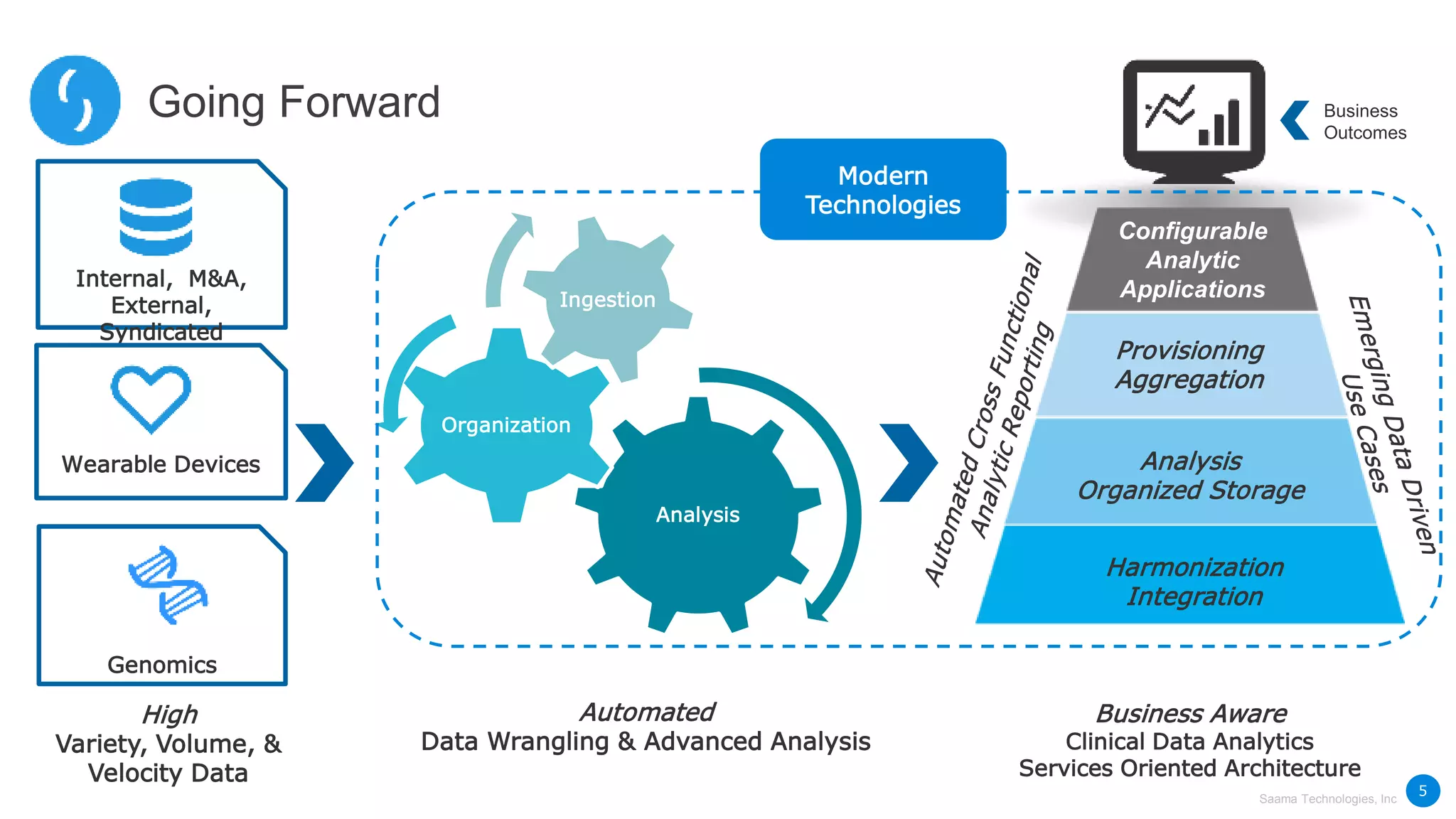
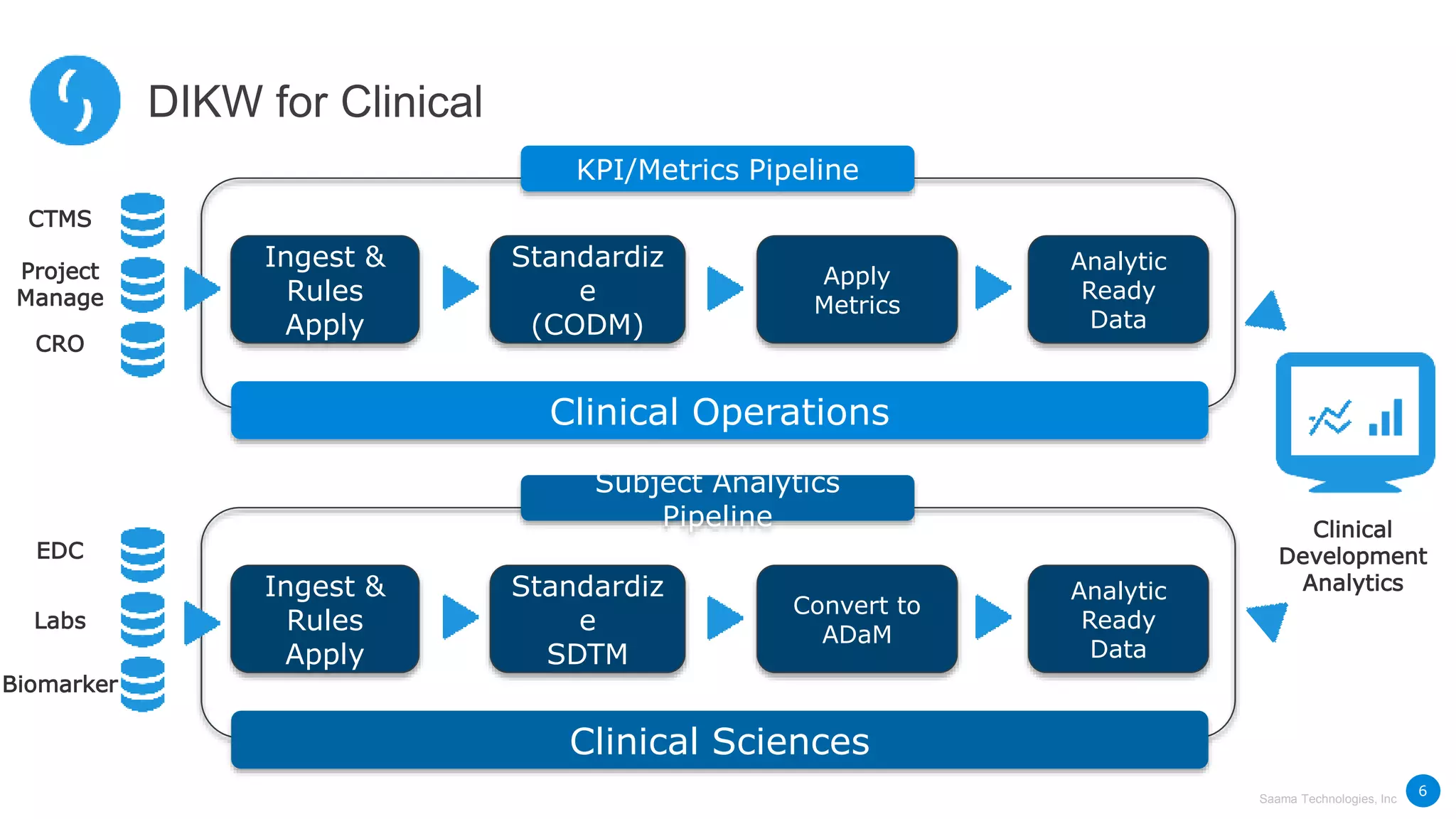
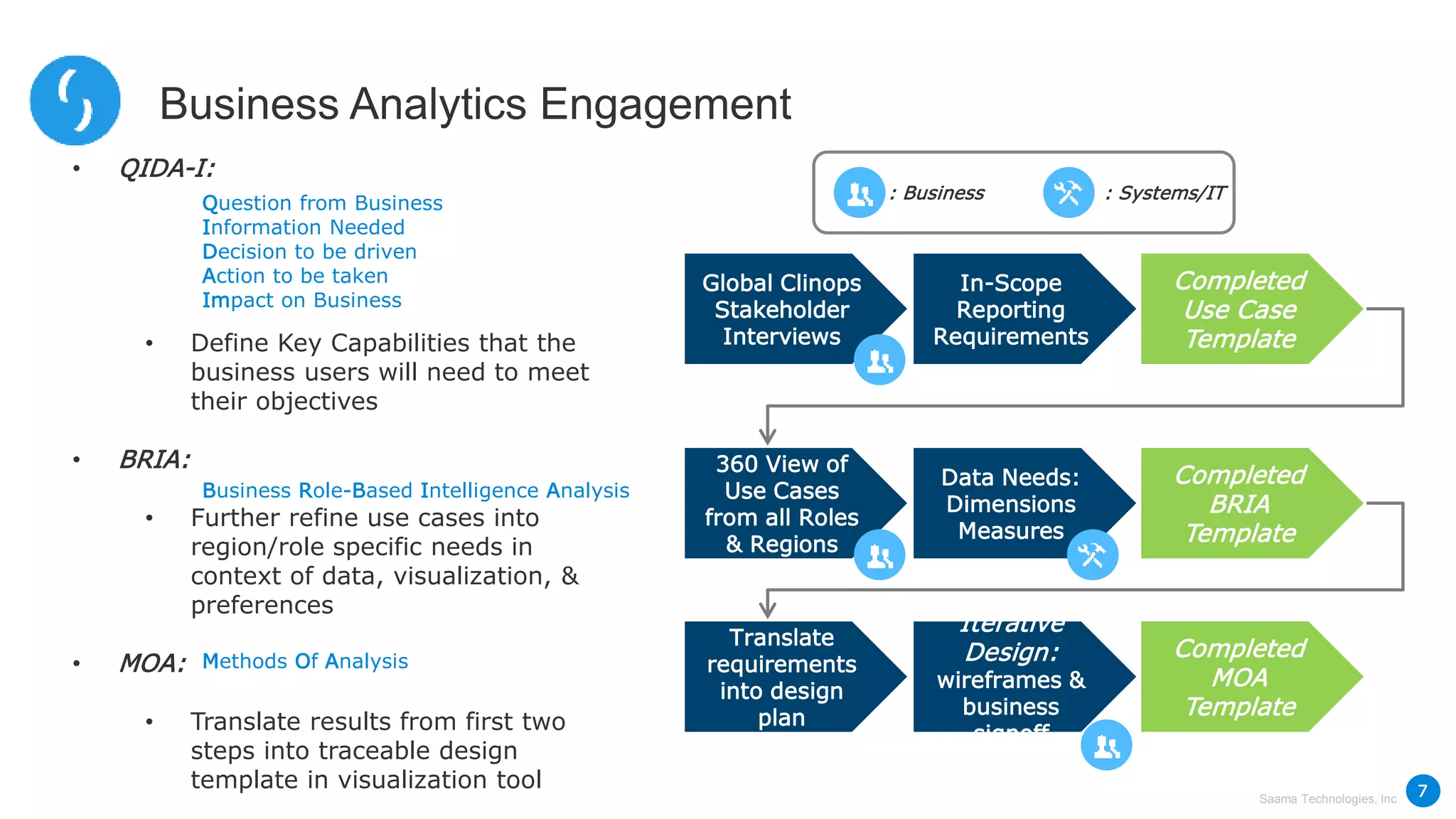
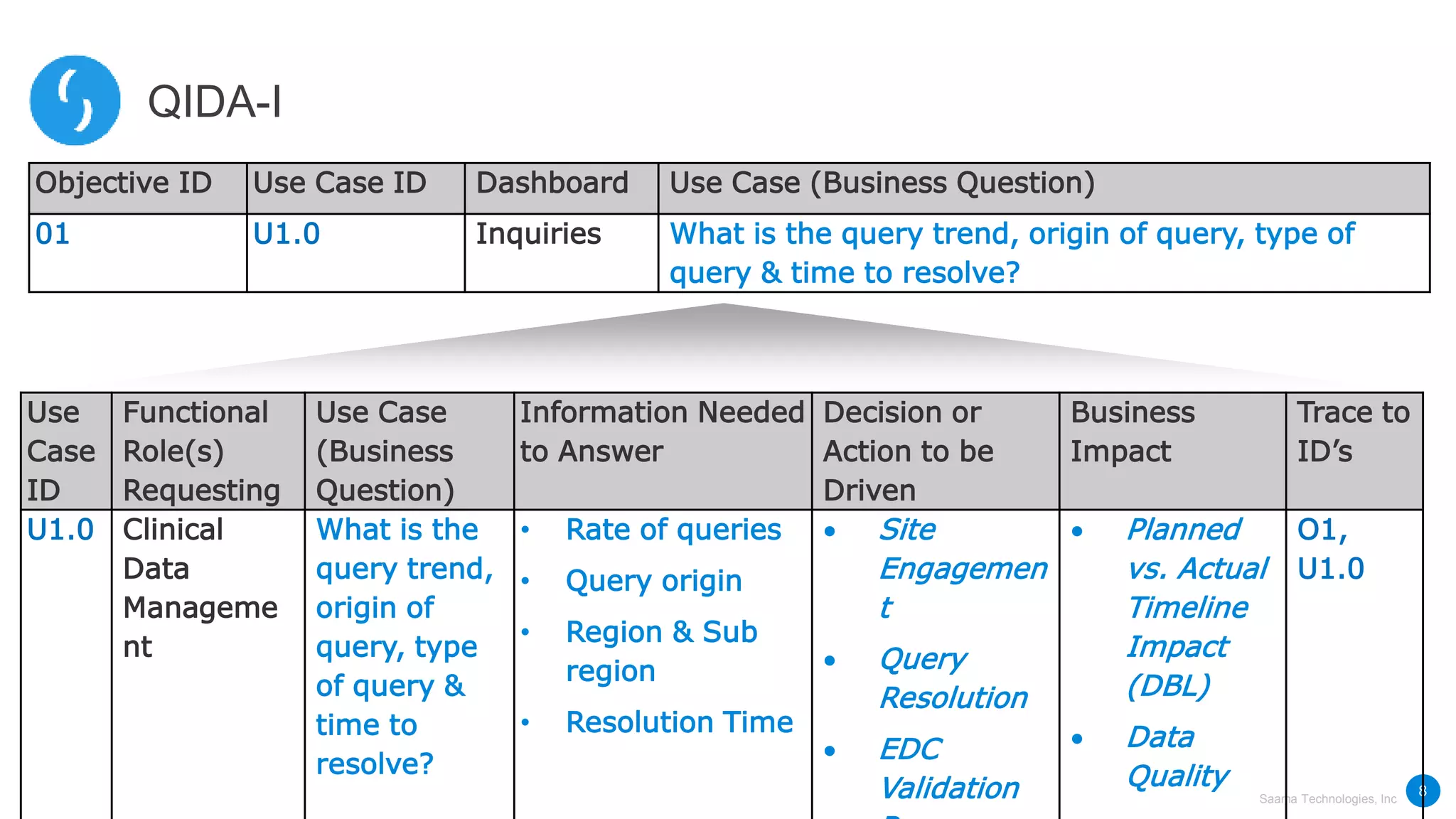
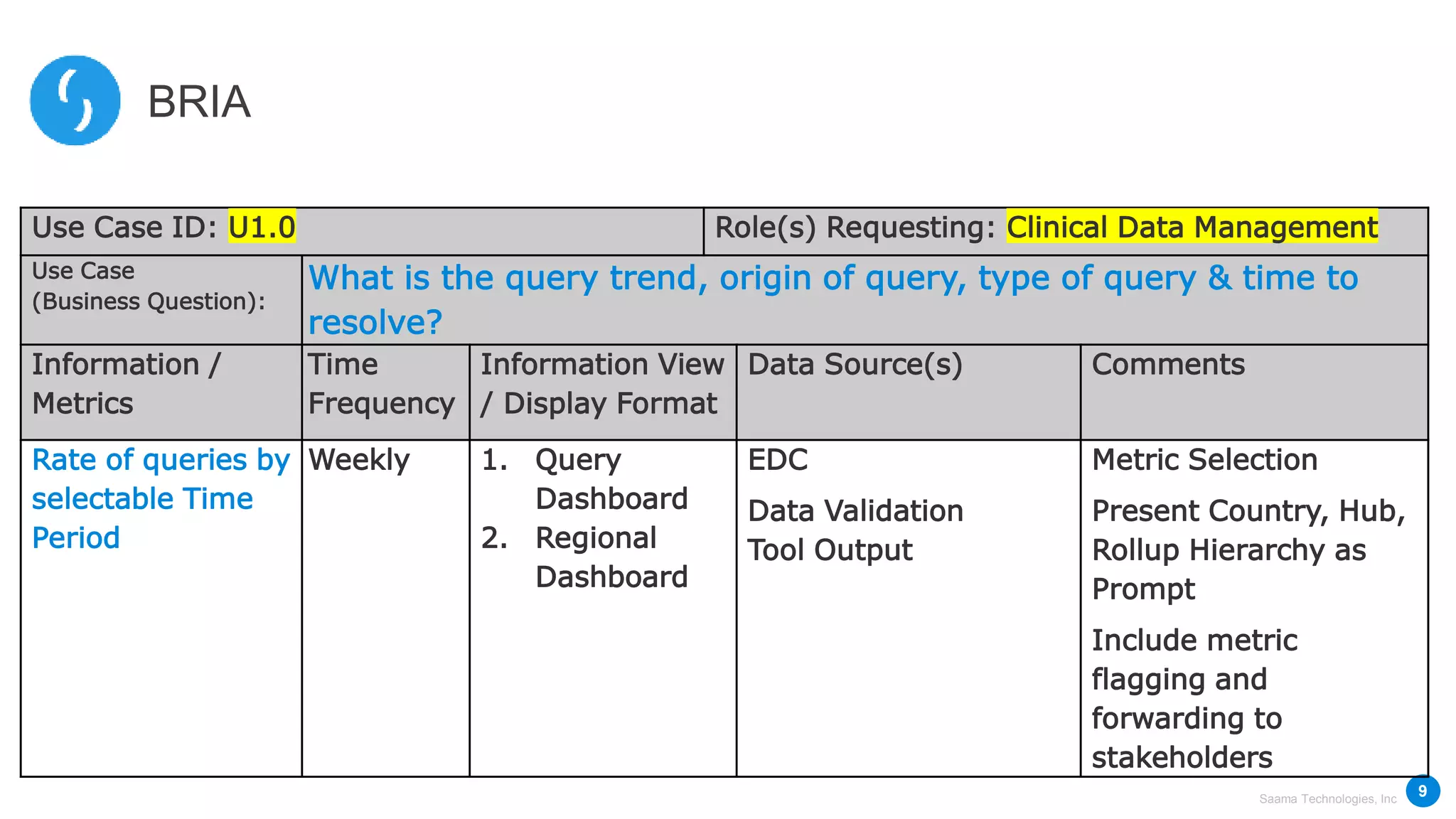
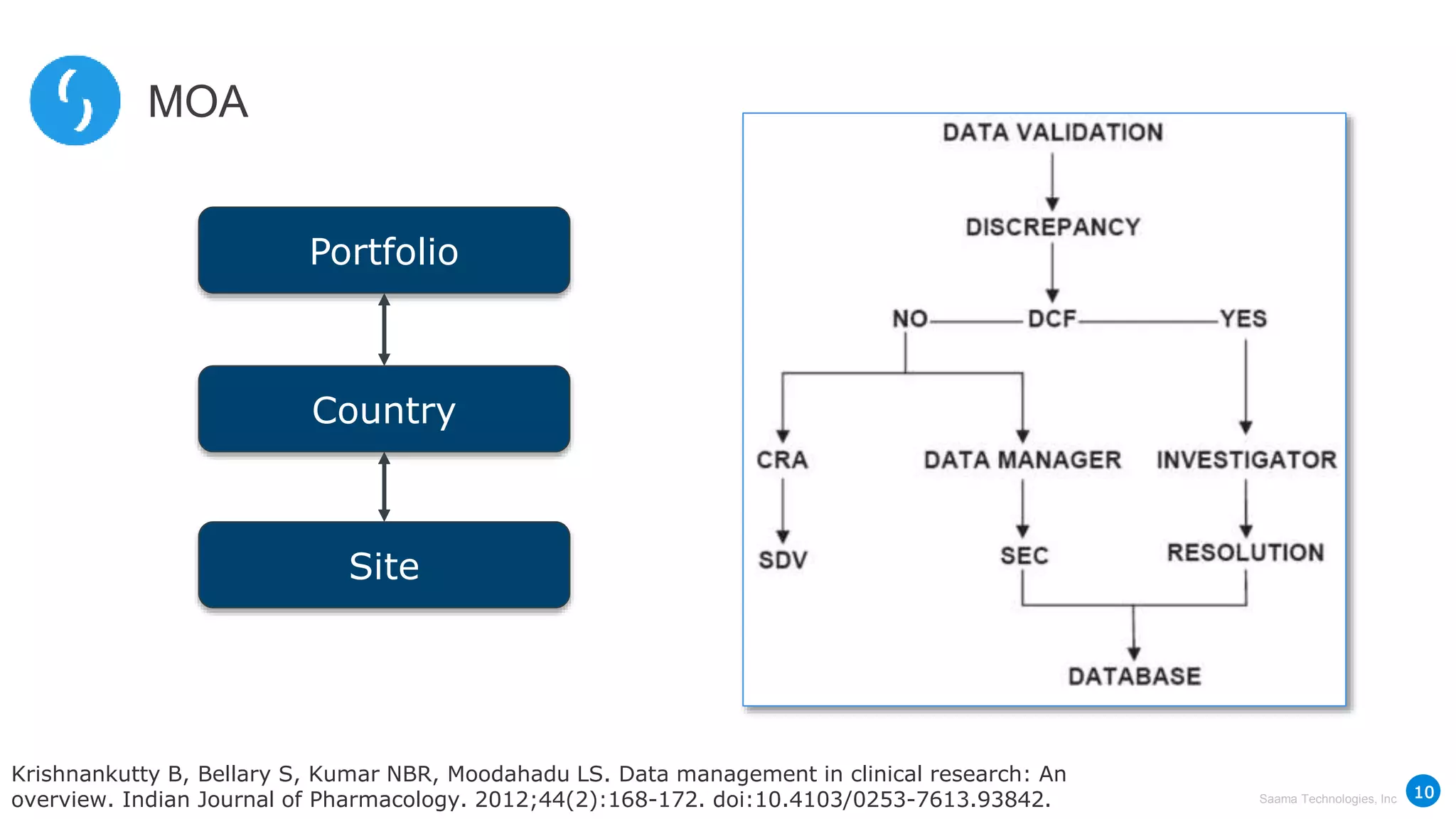
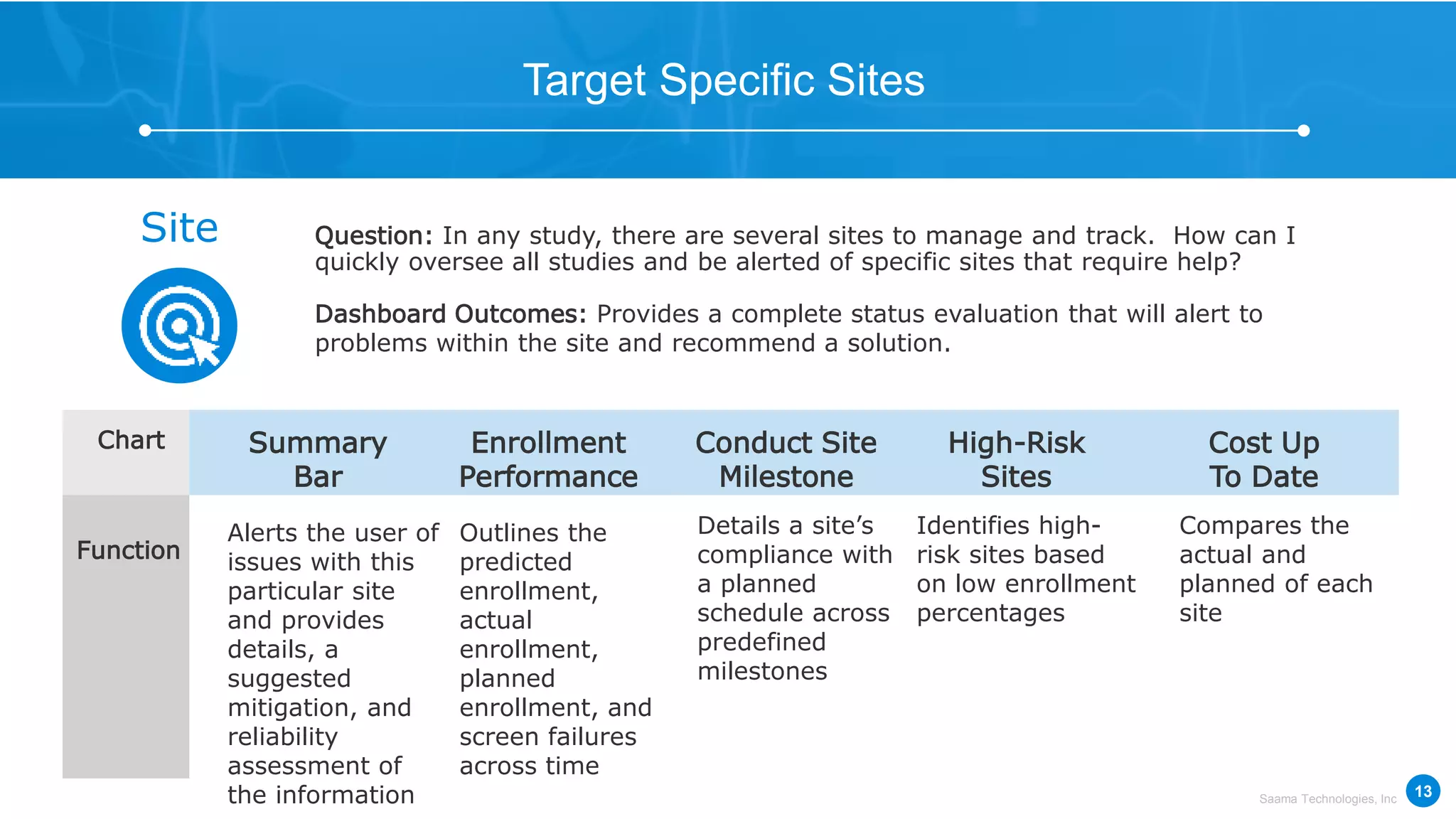
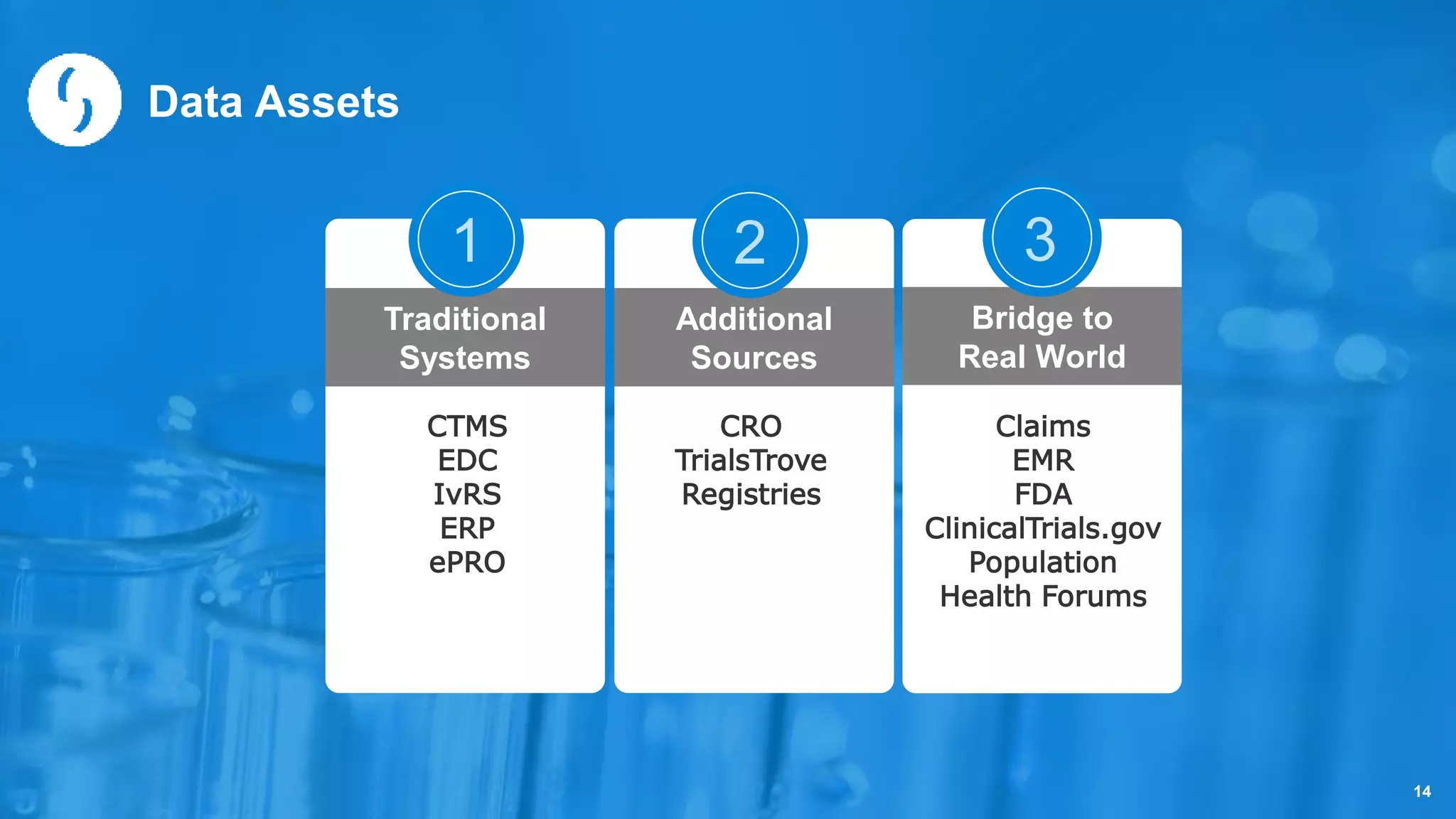
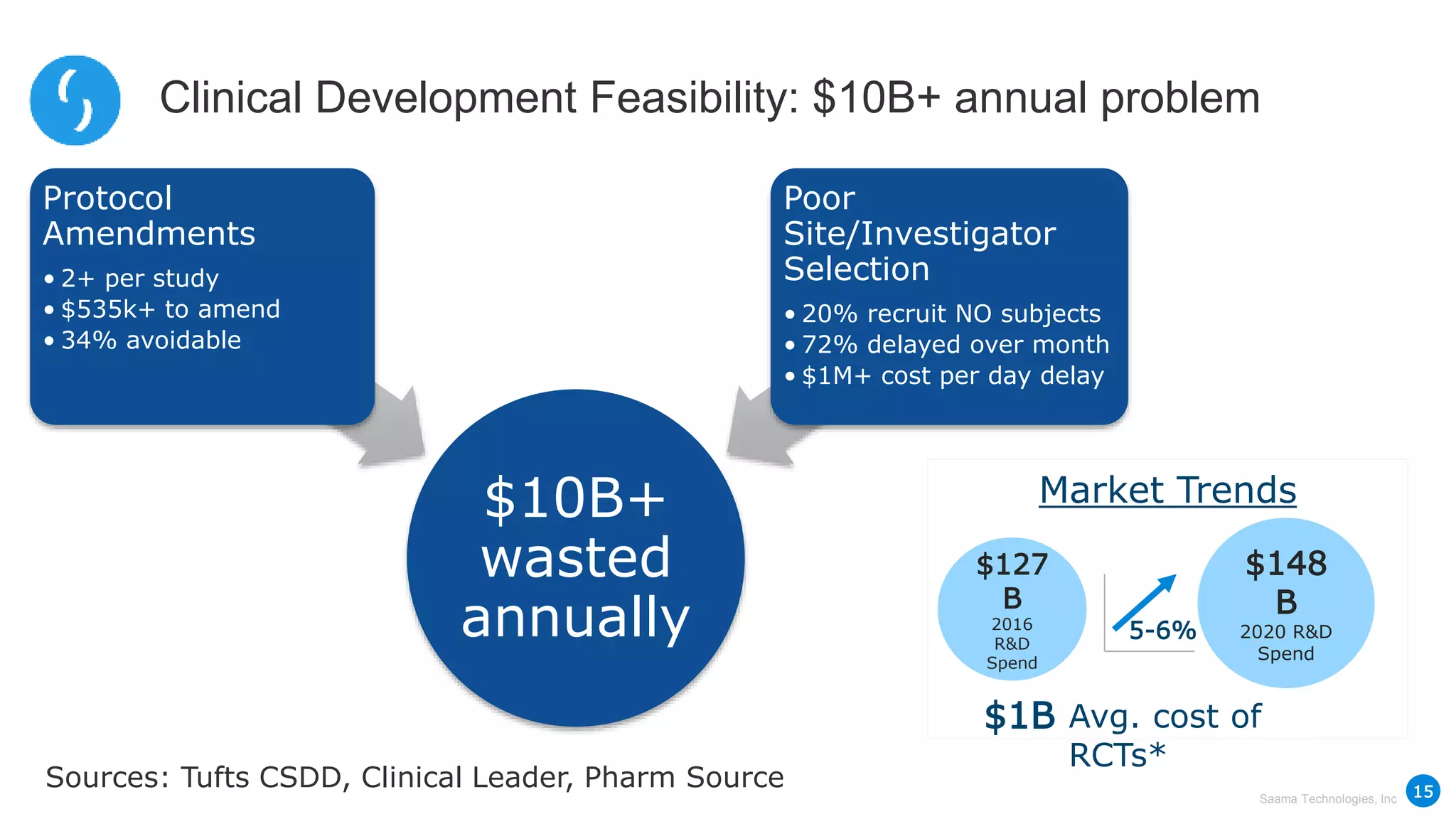
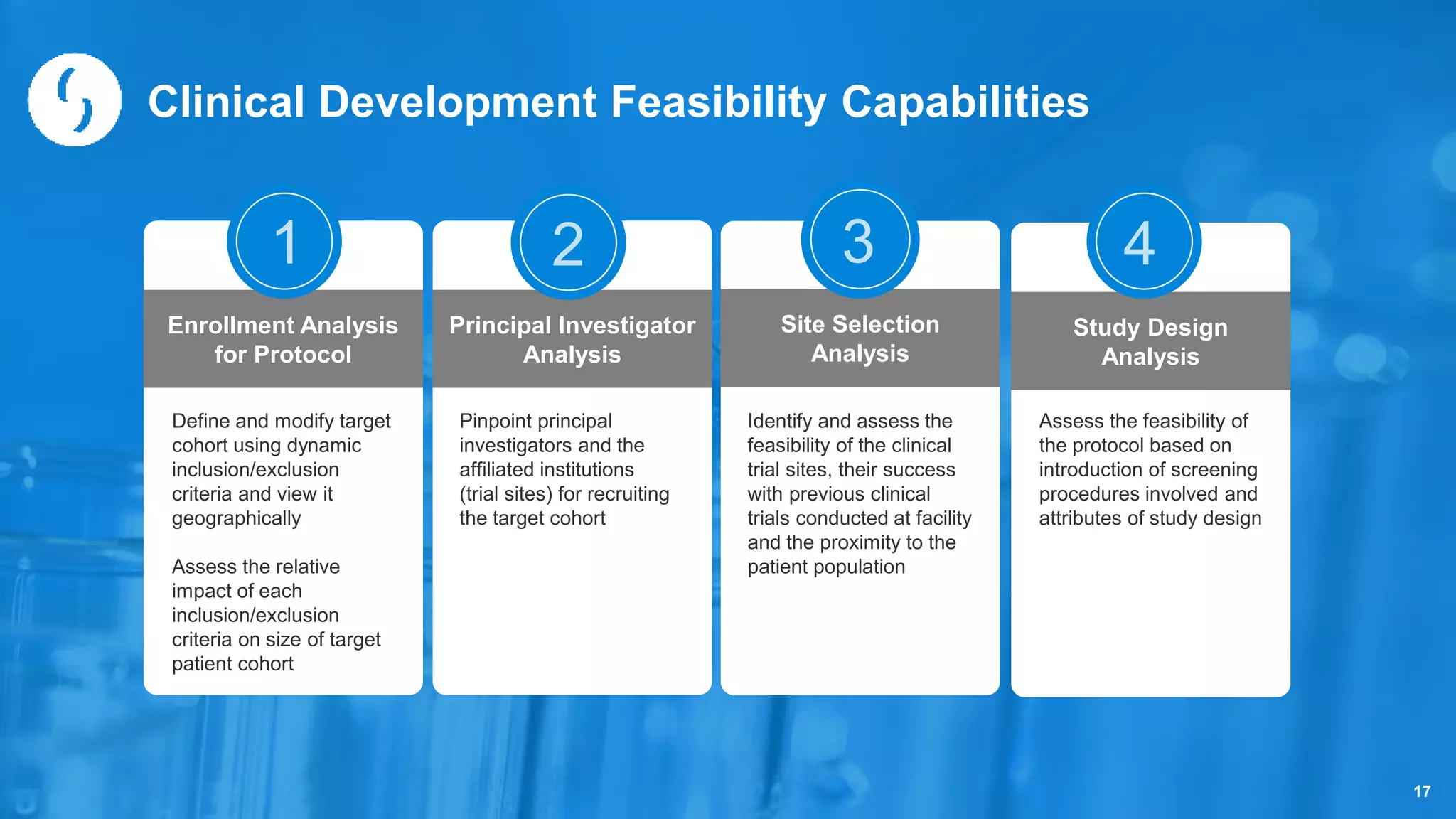
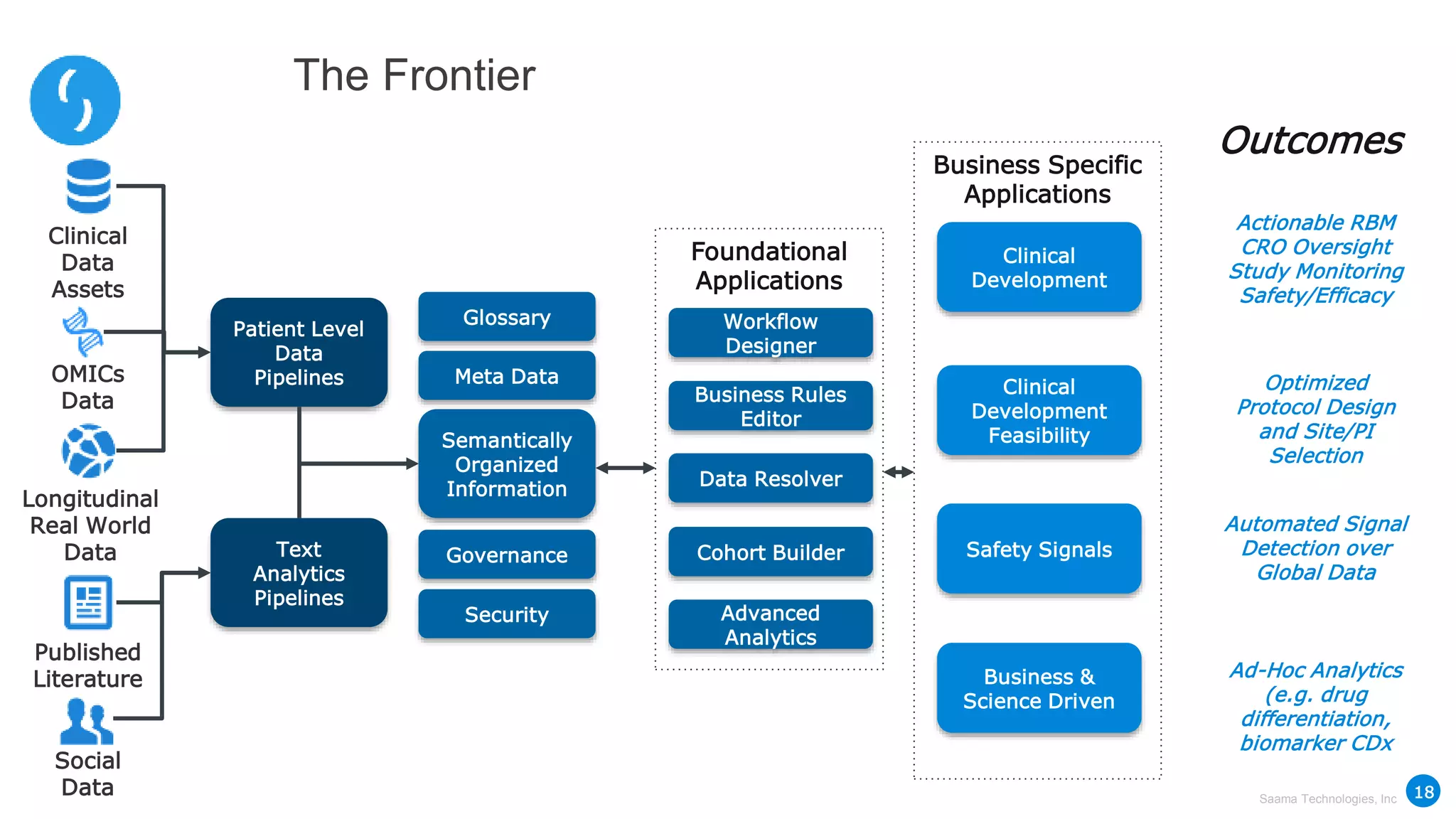
Saama Technologies, Inc. utilizes big data analytics to improve clinical trial management, from planning to execution, by integrating various data sources and delivering predictive insights. The document outlines frameworks for managing clinical data, analyzing trends, and optimizing trial designs, thereby addressing common challenges in clinical research such as delays and inefficiencies. It emphasizes the importance of real-world data and intelligent applications in enhancing decision-making and operational outcomes in clinical trials.