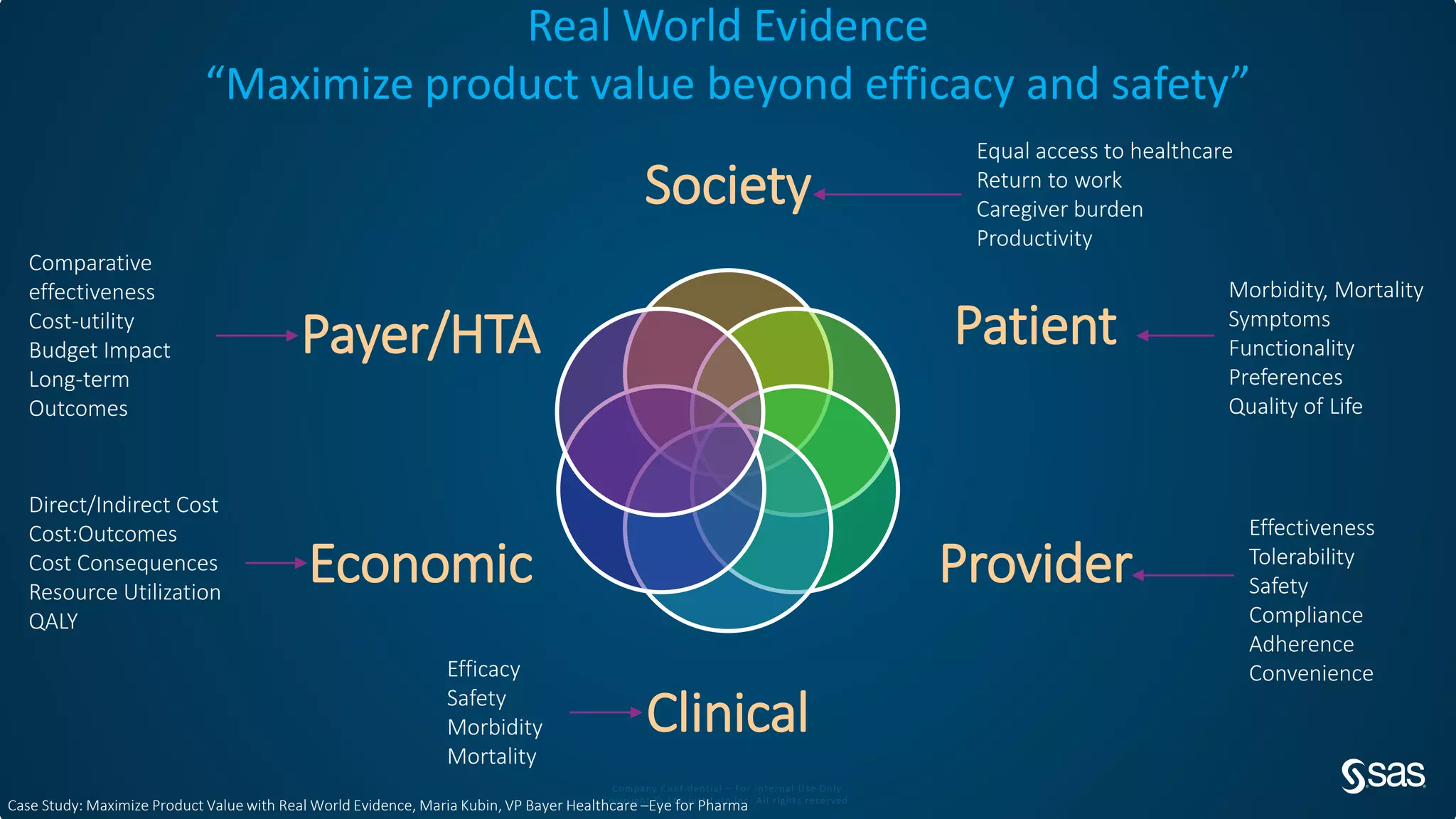
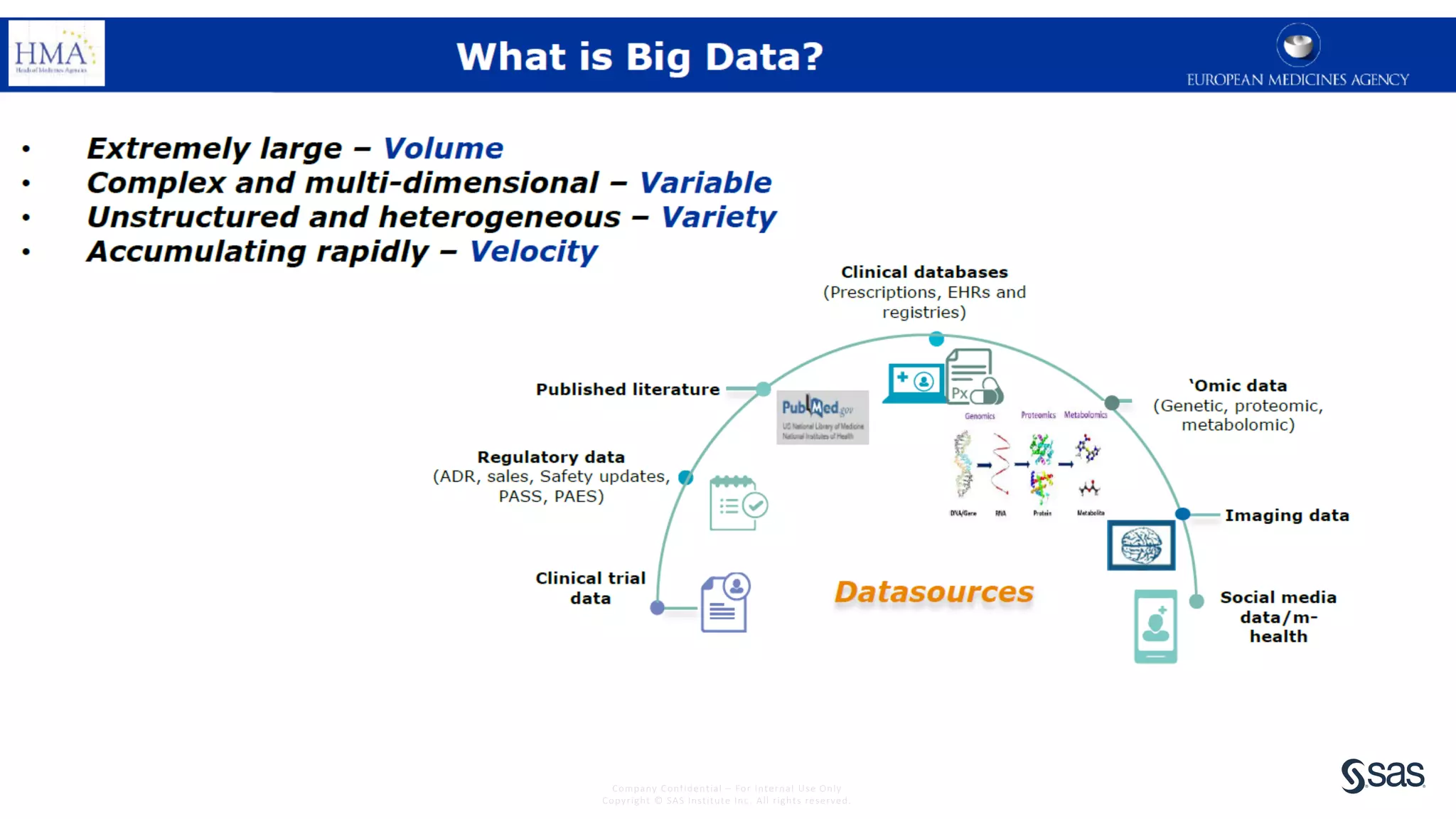
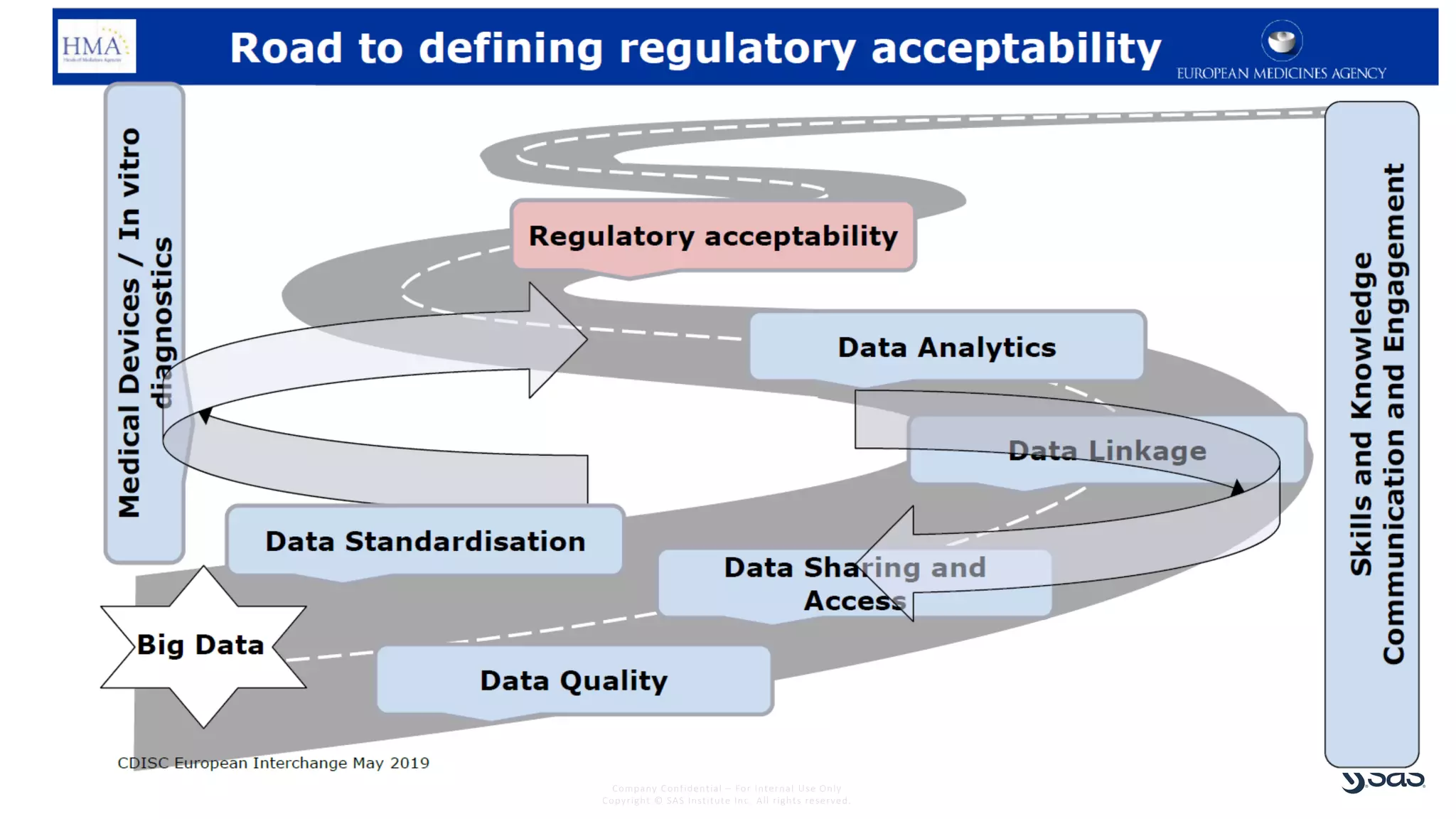
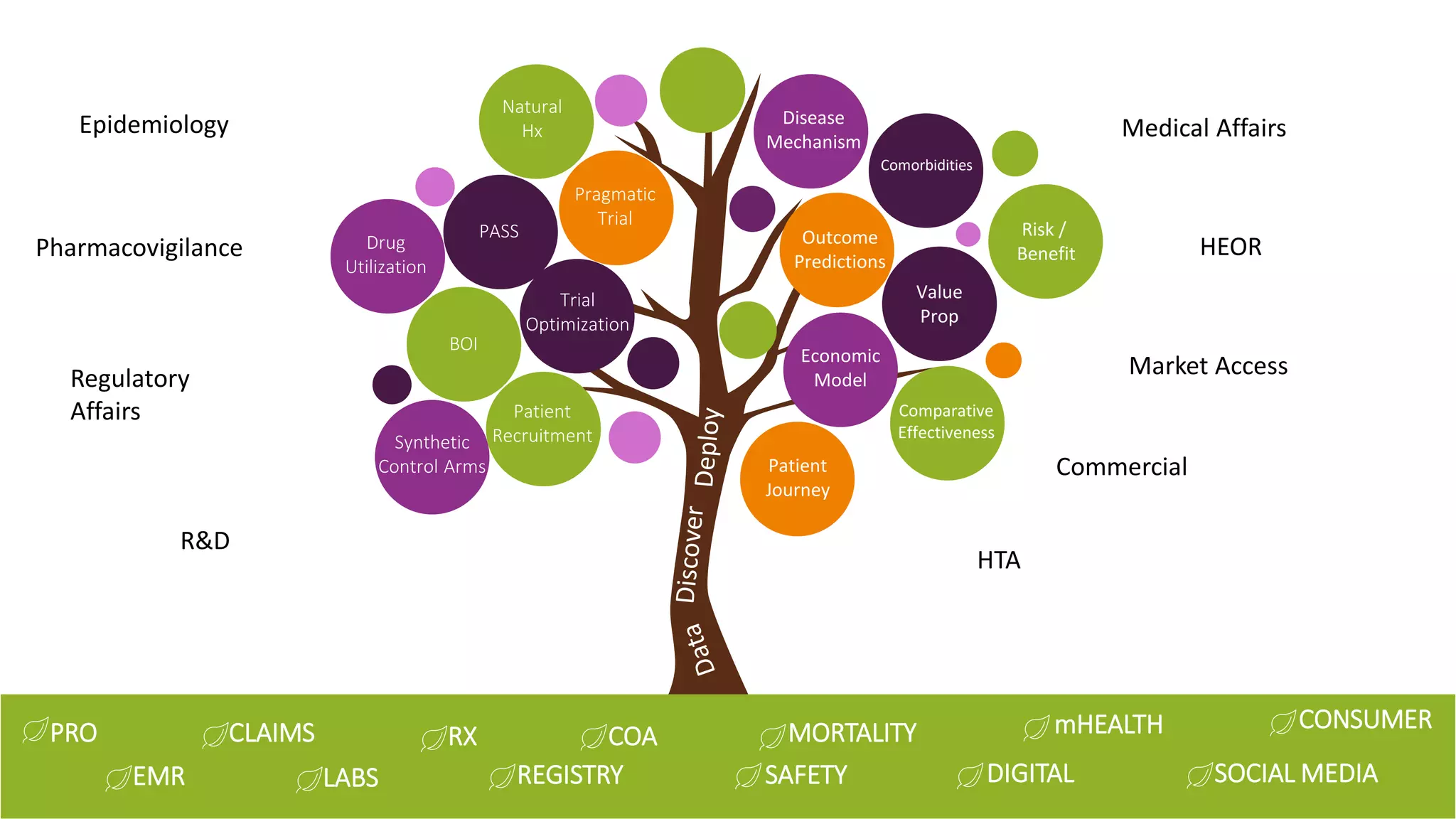
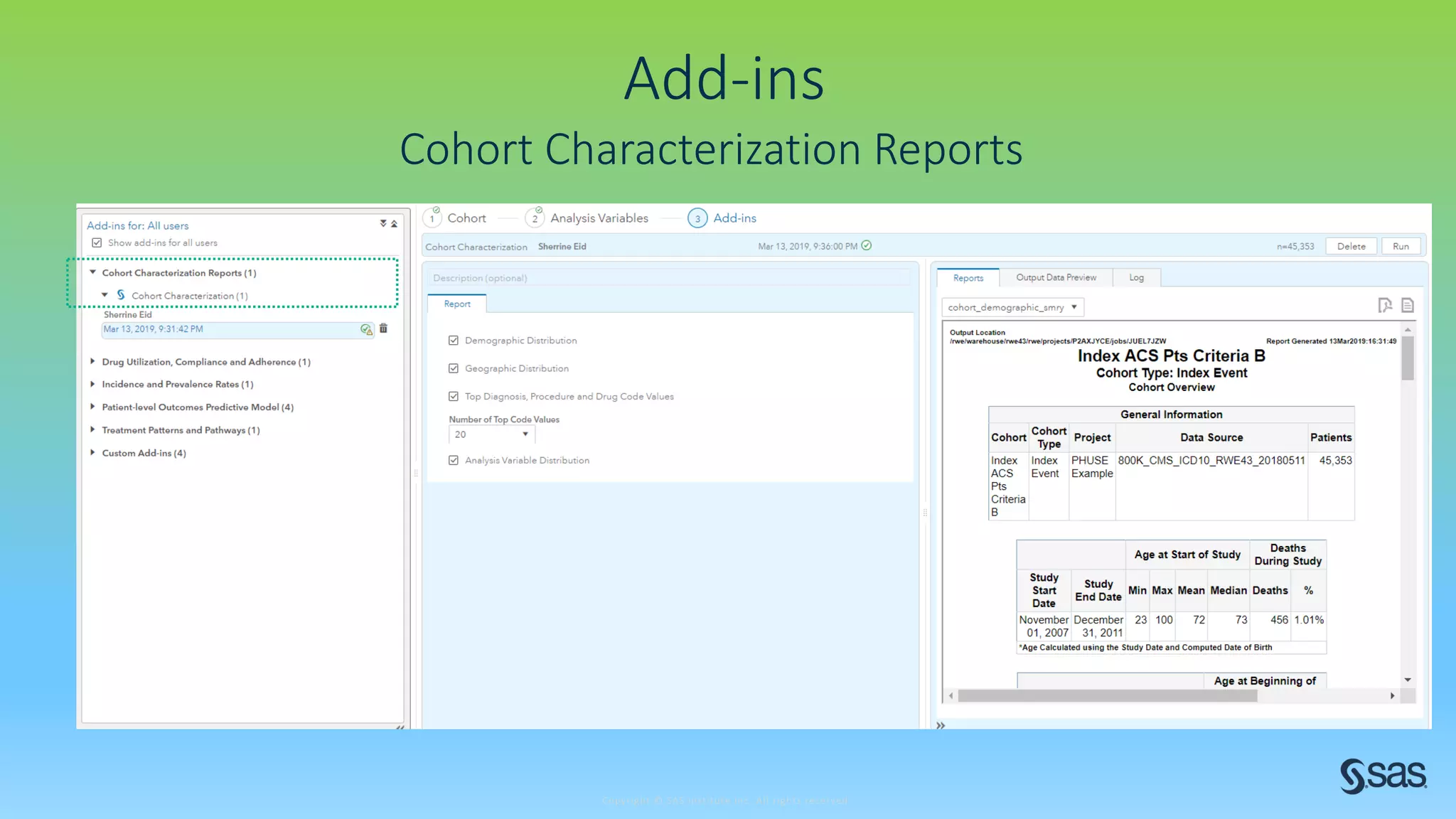
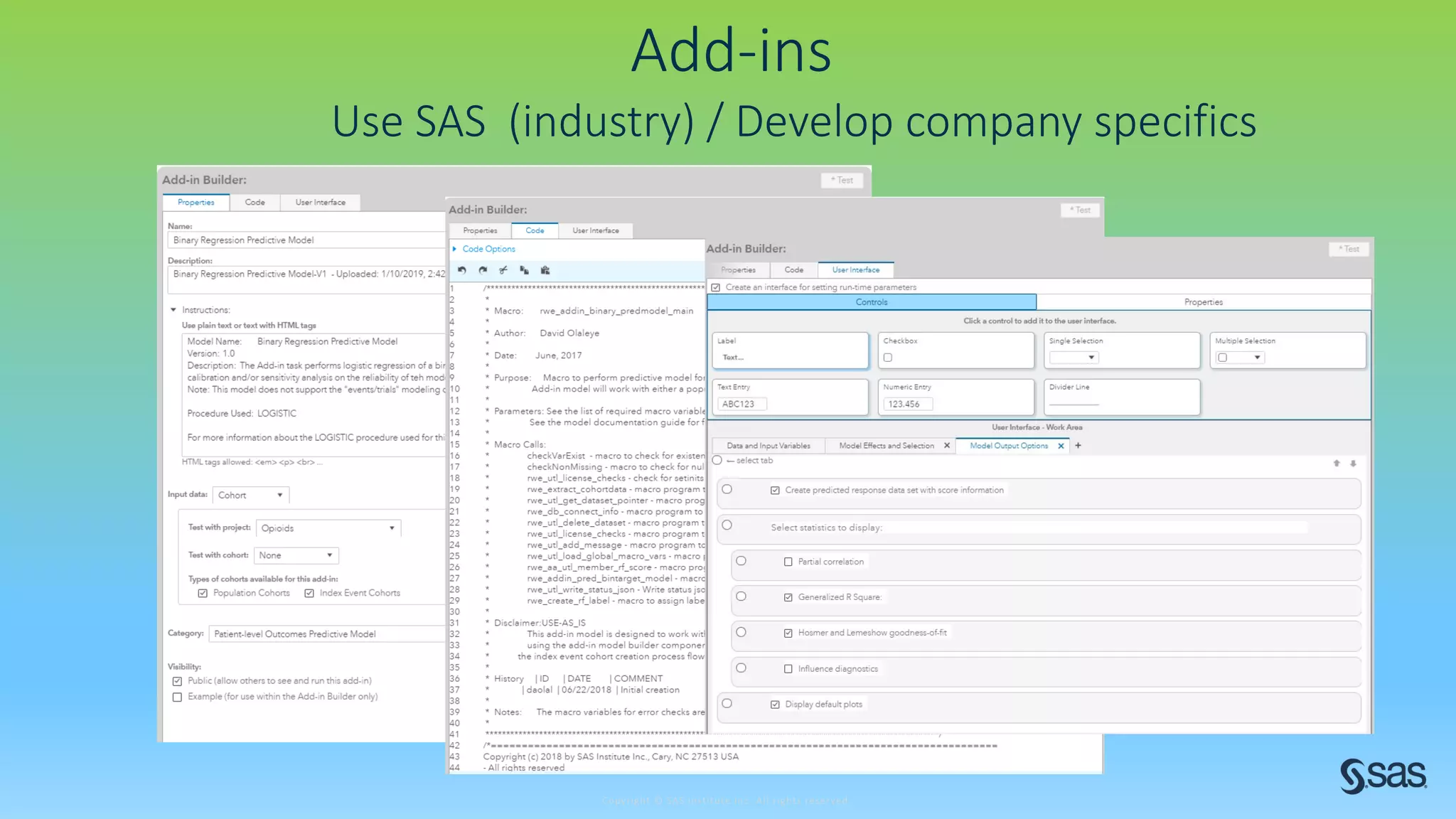
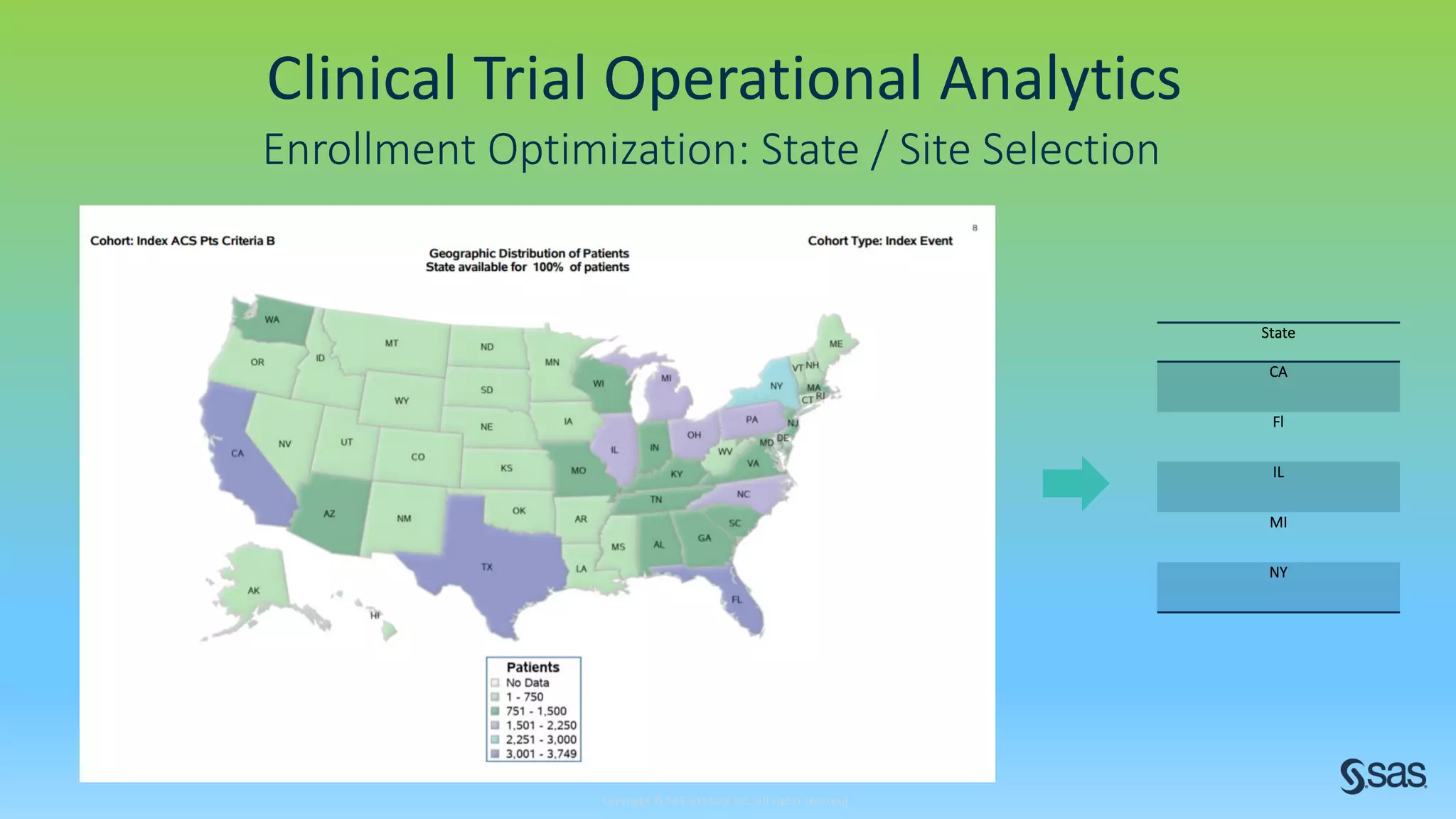
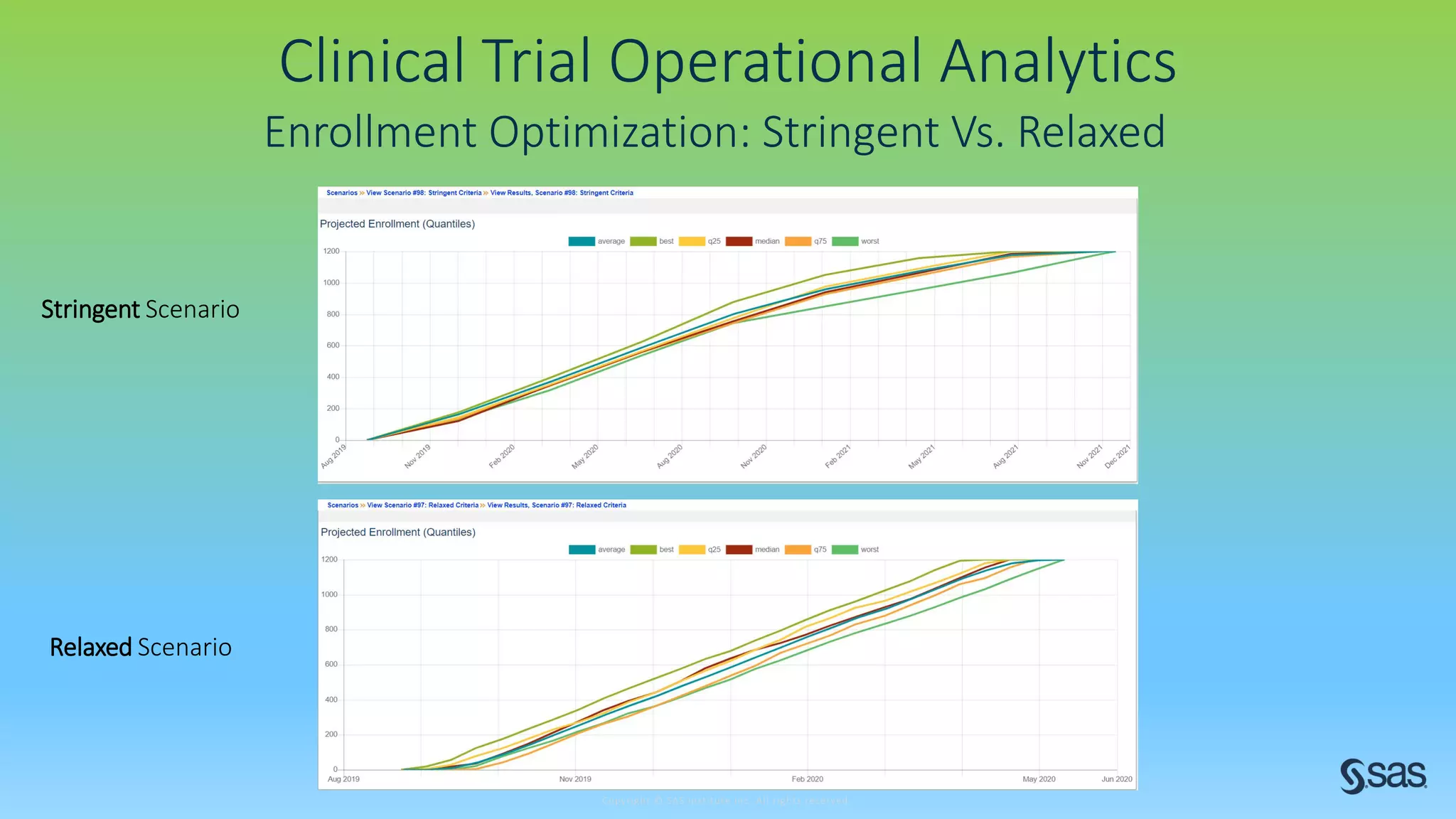
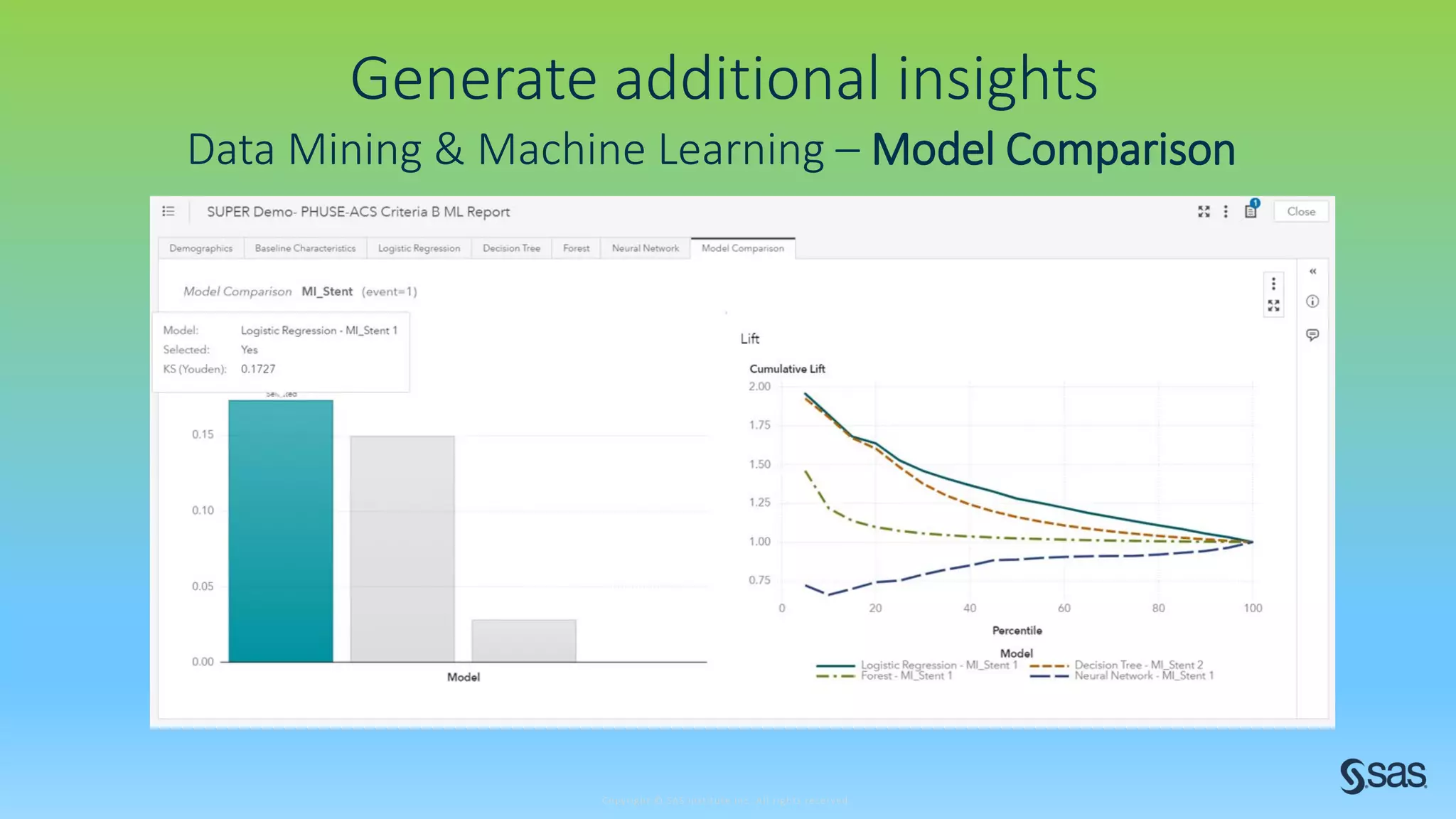
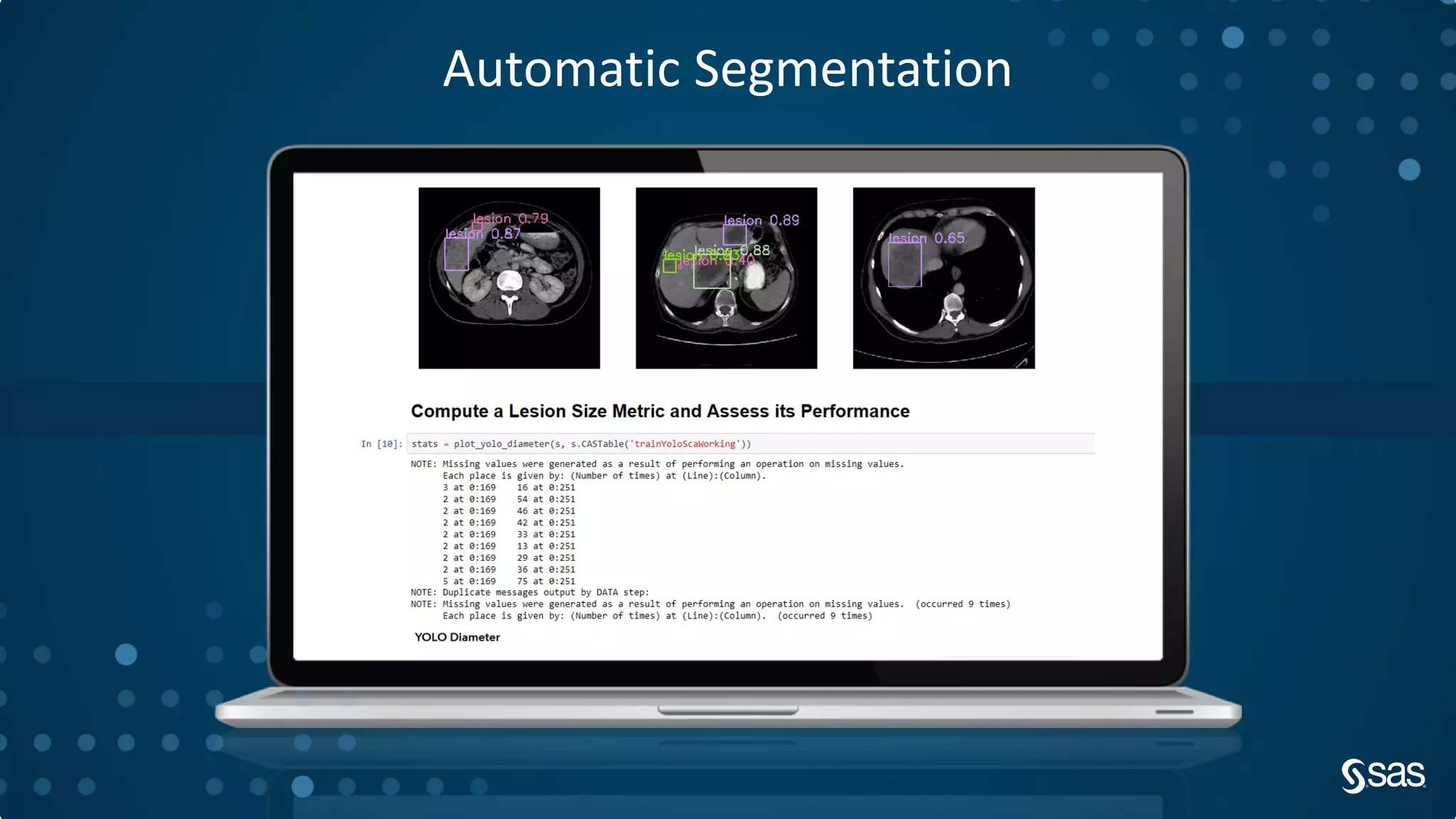
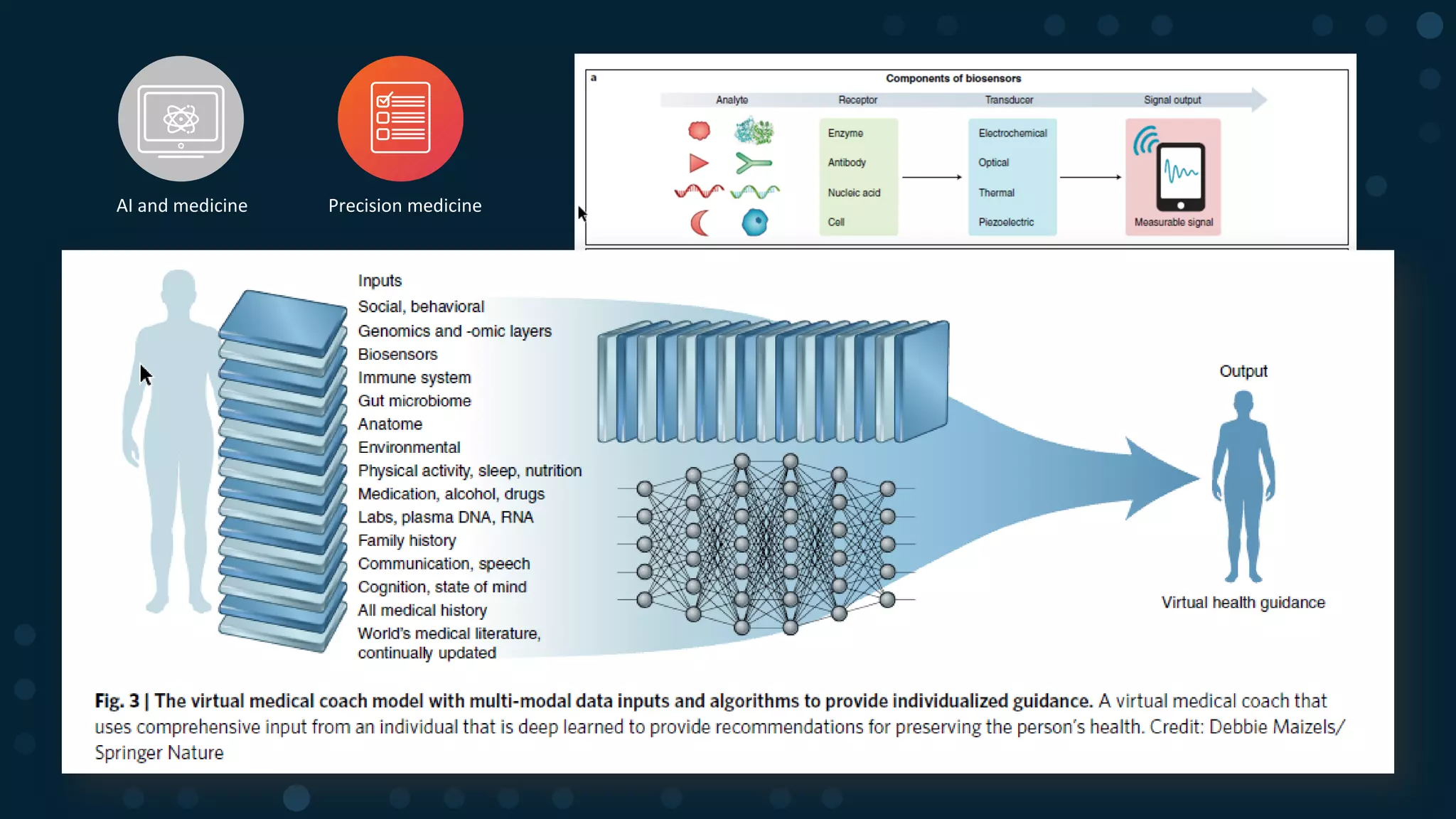
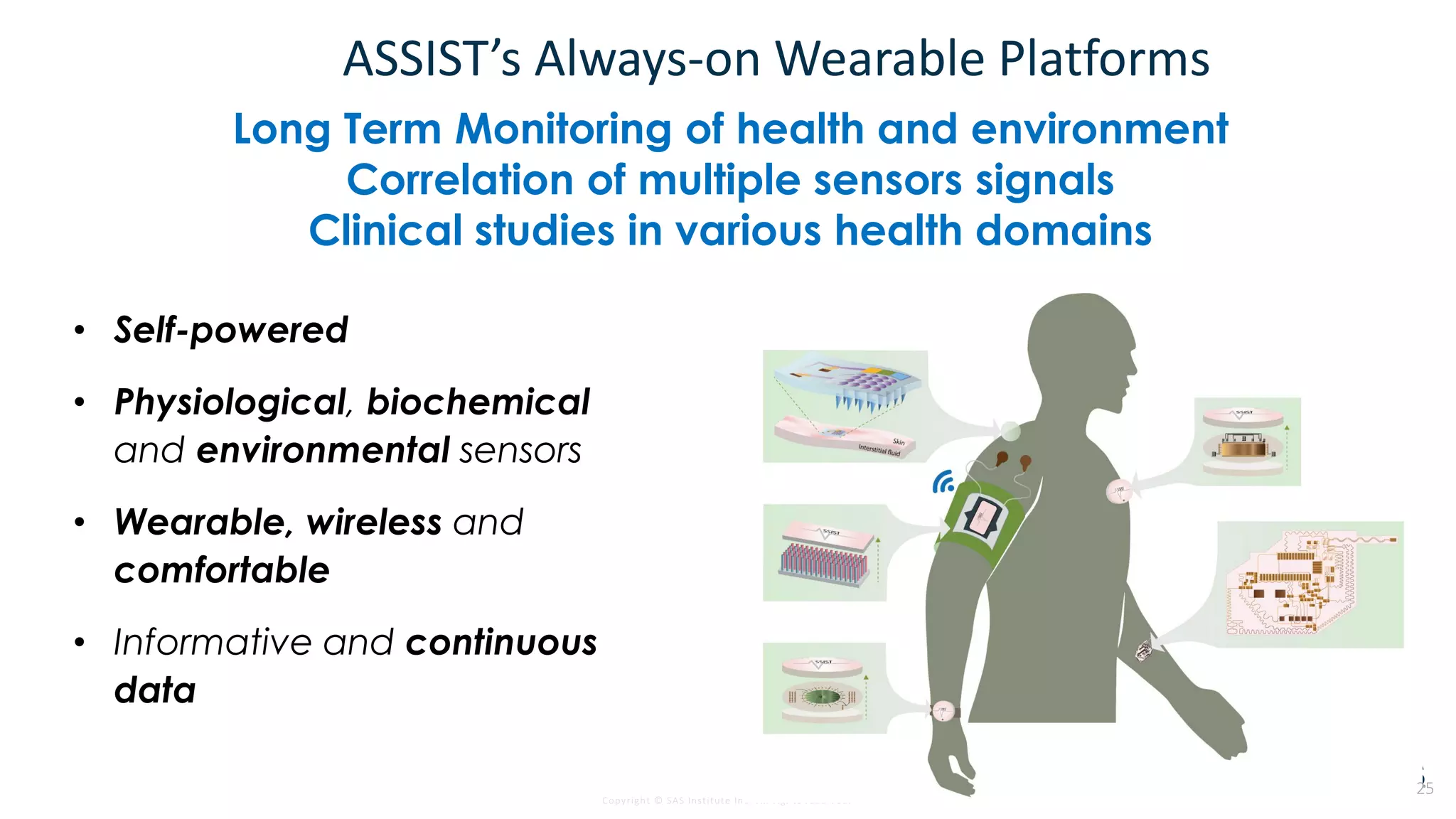
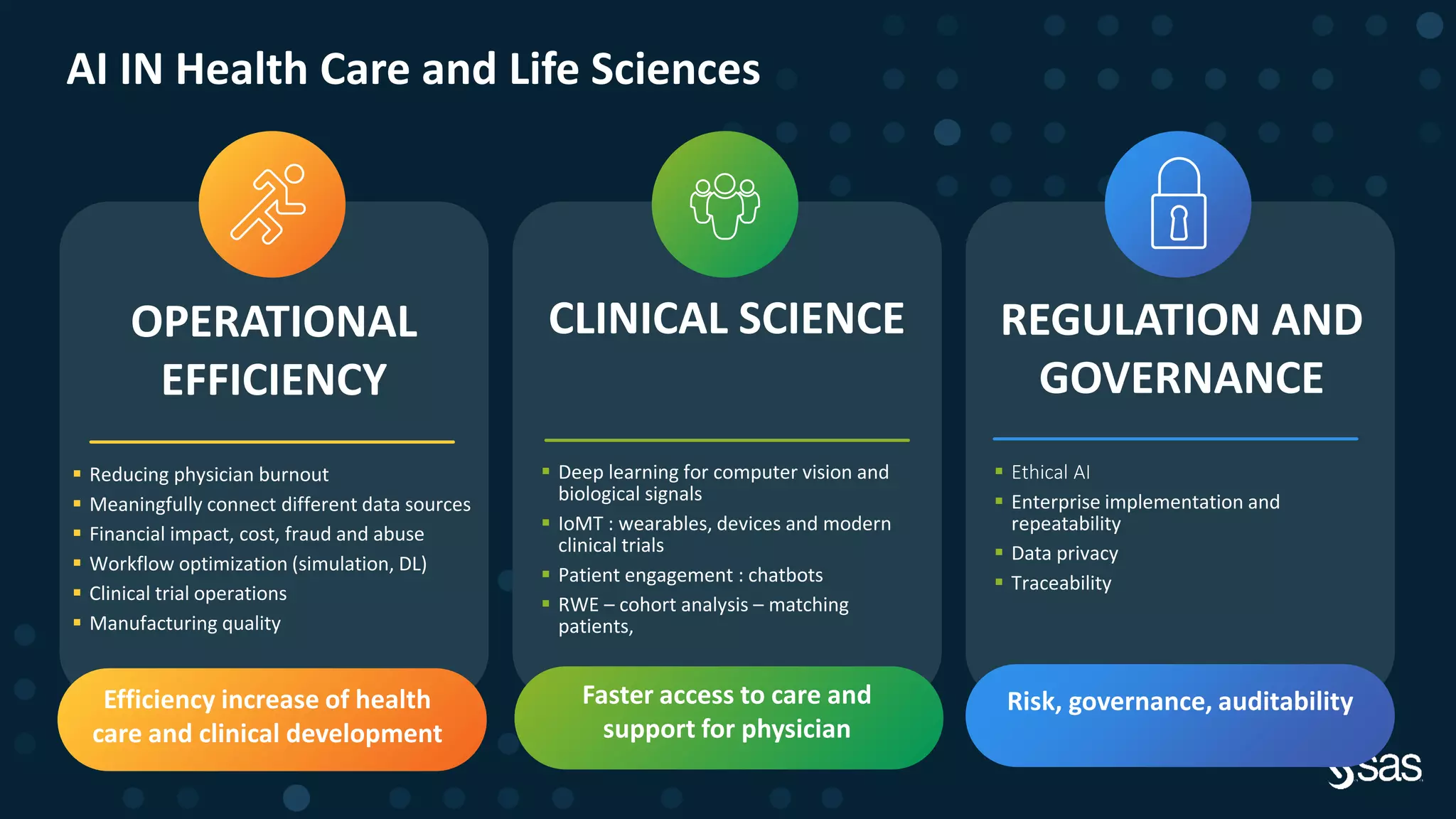
The document discusses the evolution of clinical trials using real-world evidence to enhance product value, focusing on regulatory perspectives and feasibility analysis for effective patient enrollment. It emphasizes the importance of incorporating advanced analytics and AI technologies for optimizing trial operations and improving outcomes. Key points include the need for relaxed eligibility criteria to enhance trial participation and foster medical innovation across various disciplines.