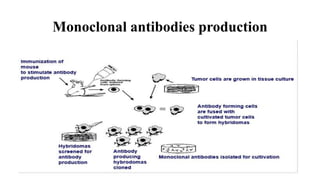
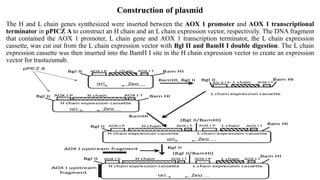
1) The document describes the construction of a plasmid to produce the monoclonal antibody Trastuzumab (Herceptin) in Pichia pastoris yeast for treatment of HER2-positive breast cancer. Genes for the heavy and light chains of Trastuzumab were optimized for expression in P. pastoris and inserted into an expression vector.
2) The vector was used to transform P. pastoris cells, which were then cultured and induced to express and secrete Trastuzumab into the culture medium.
3) The expressed Trastuzumab was characterized, affinity purified using a protein A column, and formulated into lyophilized vials for therapeutic use, with each vial containing 150 mg



![Production of Trastuzumab
• P. pastoris conaining the trastuzumab expression vector was cultured from in
10 mL of BMGY medium [1% yeast extract, 2% peptone, 1.34% yeast
nitrogen base, 100 mM potassium phosphate (pH 6.0), 4 × 10–5% biotin, and
1% glycerol] overnight at 30˚C with shaking at 275 rpm.
• A 100-mL volume of BMGY medium was inoculated with 1 ml of the starting
culture. The second culture was incubated for approximately 12 h at 30˚C
with shaking at 275 rpm. Cells were harvested at an OD600nm of between 1
and 4 and pelleted by centrifugation for 10 min at 5000 rpm at 4˚C.](https://image.slidesharecdn.com/trastuzumab-final1-191101145129/85/Trastuzumab-8-320.jpg)
![• Induction of protein expression was initiated by resuspending the yeast
cells in 100 mL of BMMY medium [1% yeast extract, 2% peptone, 1.34%
yeast nitrogen base, 100 mM potassium phosphate (pH 6.0), 4 × 10−5 %
biotin, and 1% methanol]. Incubation was continued at 30˚C with shaking
at 300 rpm for 24 h. A supplemental volume of methanol equal to 1/100
of the culture volume was added at 16 h.
• Following induction for 24 h, the culture was chilled on ice, and the cells
and culture medium were separated by centrifugation for 10 min at 5000
rpm at 4˚C. The culture medium was stored at 4˚C.](https://image.slidesharecdn.com/trastuzumab-final1-191101145129/85/Trastuzumab-9-320.jpg)