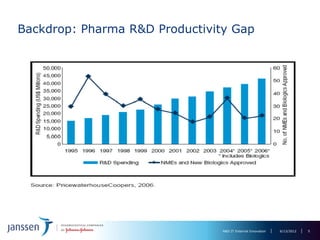
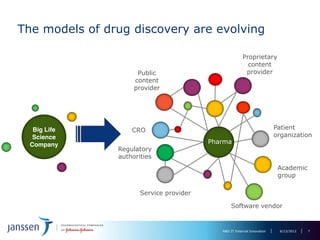
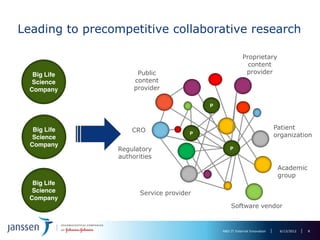
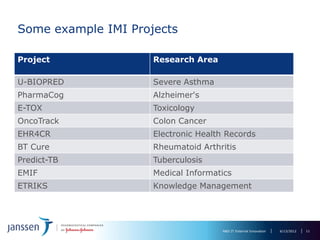
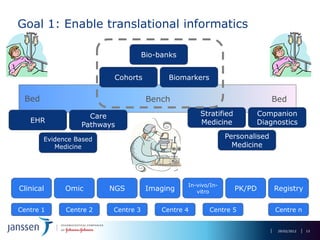
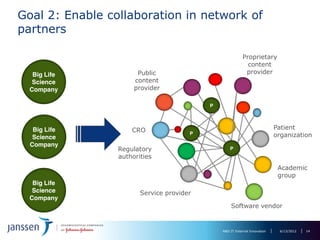
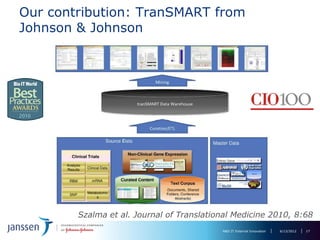
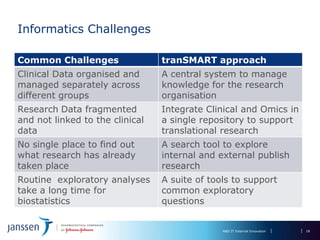
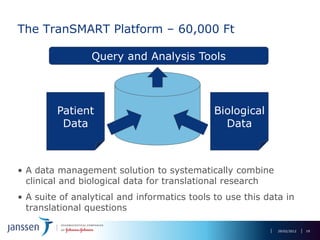
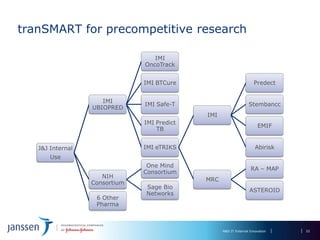
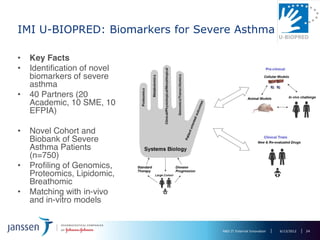
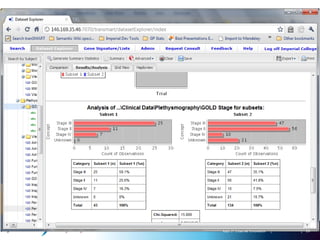
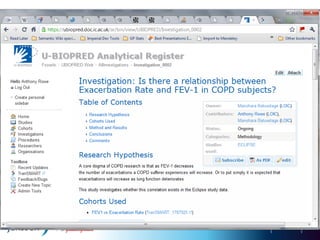
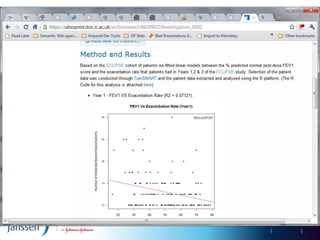
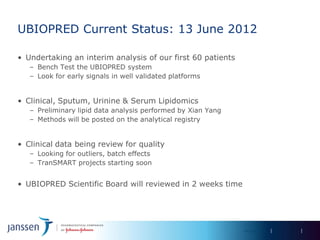
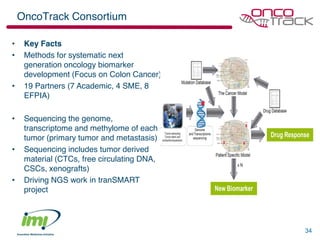
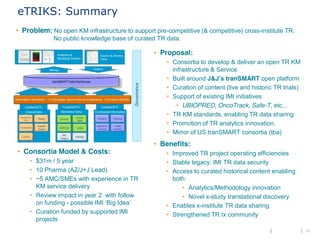
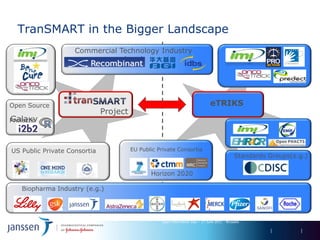
The document discusses the concept of pre-competitive research in pharmaceutical R&D, emphasizing the need for collaborative frameworks and innovative informatics solutions, exemplified by the transmart platform. It highlights various ongoing projects and partnerships aimed at integrating clinical and biological data for translational research. The transmart initiative aims to facilitate data sharing and collaboration among pharmaceutical companies and academic institutions to enhance research efficiency and outcomes.