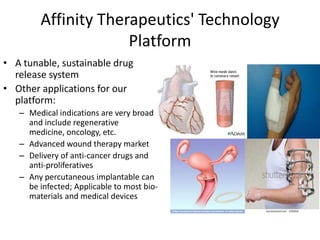
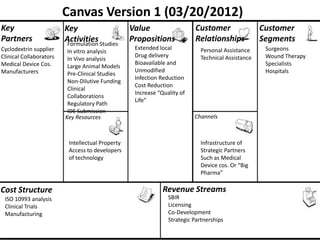
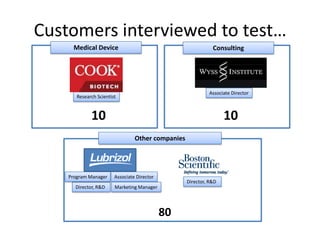
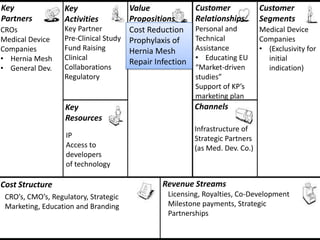
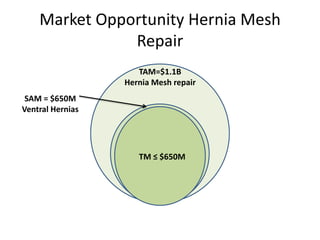
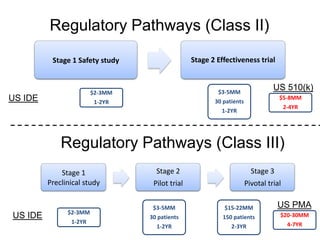
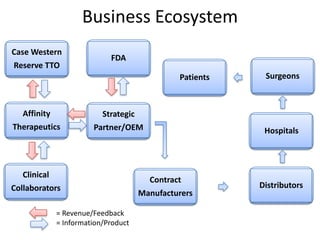
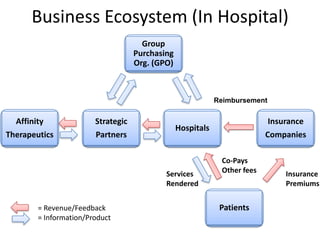
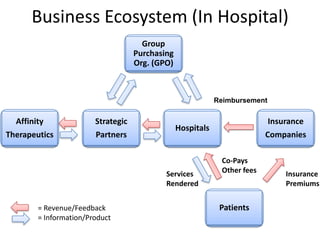
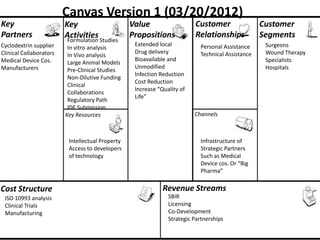
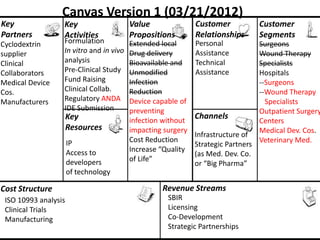
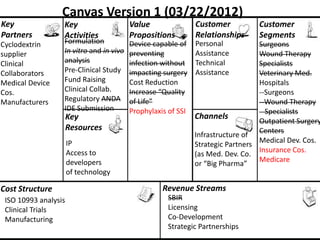
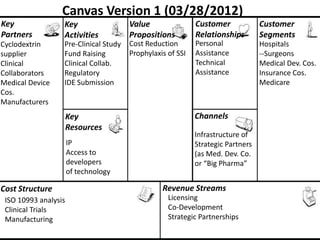
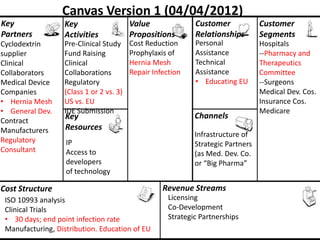
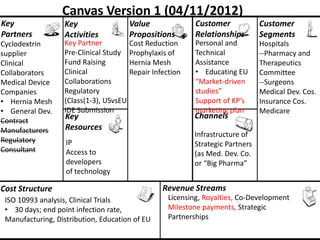
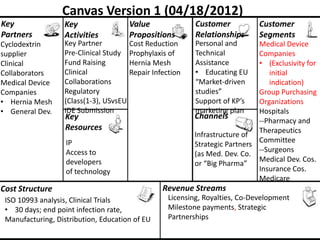
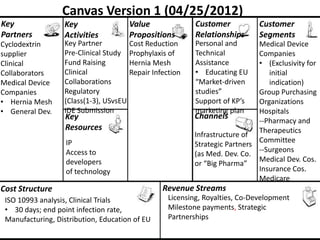
The document discusses Affinity Therapeutics' technology platform for developing microparticle formulations that provide extended local release of antibiotics to prevent surgical site infections. The company was founded in 2010 and is developing formulations to be used with hernia mesh repairs initially, with the goal of licensing the technology to medical device companies. The company has received an NIH Phase I SBIR grant and is working to negotiate licensing and facility agreements with universities.