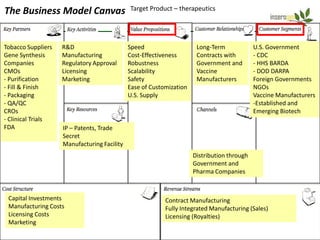
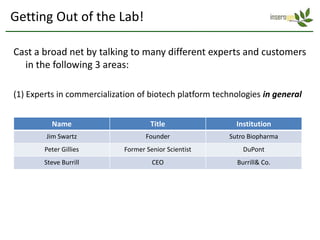
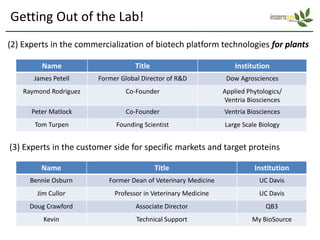
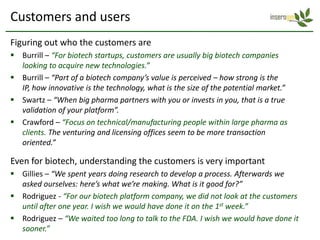
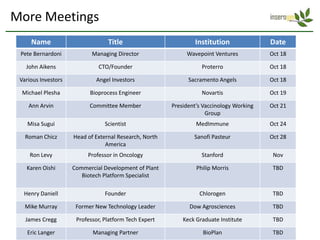
The document discusses a manufacturing platform for producing therapeutics in tobacco plants. It summarizes meetings with experts on commercializing biotech platforms and understanding customers. Experts advised validating the platform by producing hard-to-make proteins, focusing on technical/manufacturing customers, and understanding regulatory issues. Customers cited interest in cost-effective production and combination vaccines. Next steps include further challenging the value proposition and better understanding commercialization and decision-making processes through additional expert meetings.