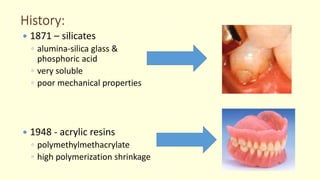
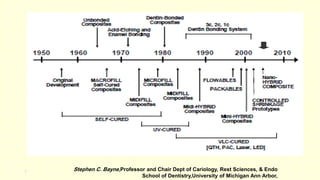
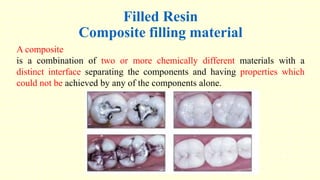
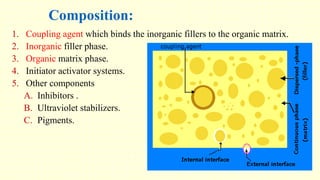
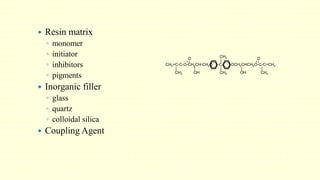
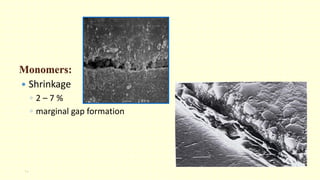
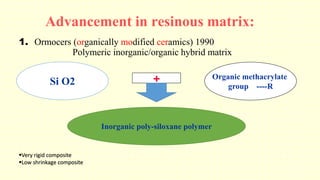
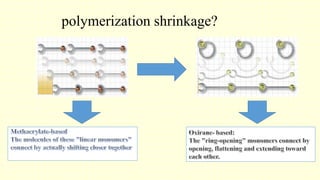
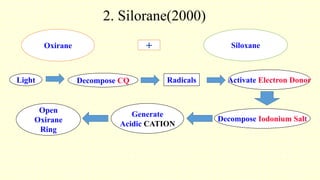
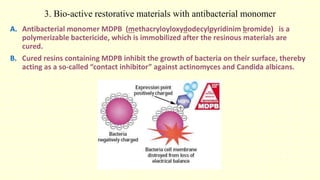
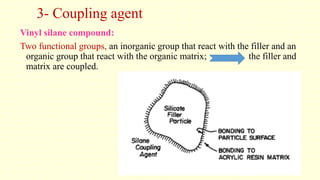
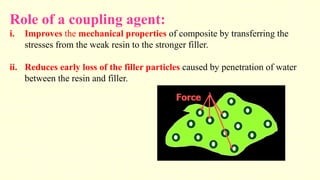
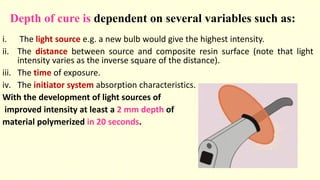
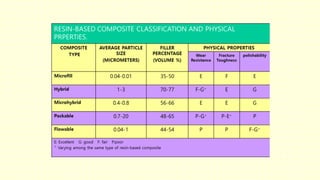
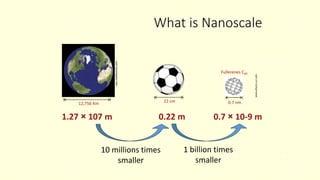
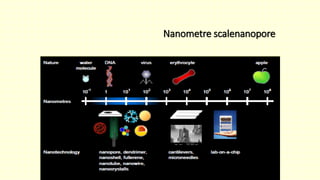
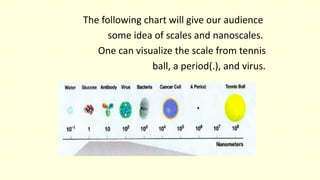
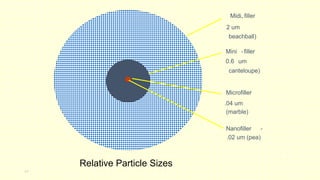
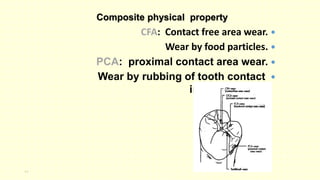
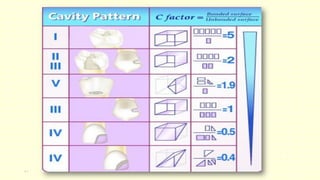
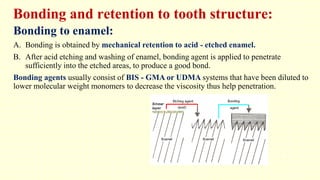
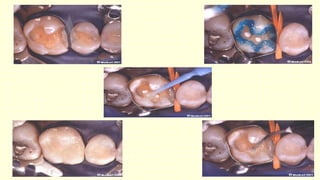
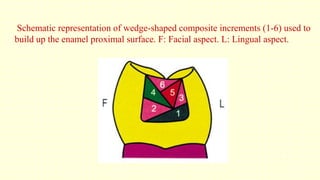
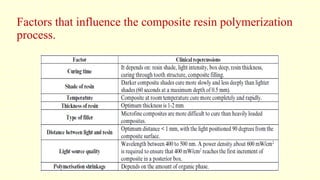
The document discusses various tooth-colored restorative materials, their historical development, and ideal requirements for effective dental applications. It details types of composites, including filled resin and their components, while emphasizing mechanical properties, bonding to tooth structure, and the importance of reducing polymerization shrinkage. Additionally, advancements in material technology, such as siloranes and antibacterial composites, are highlighted as significant improvements in dental restorative practices.