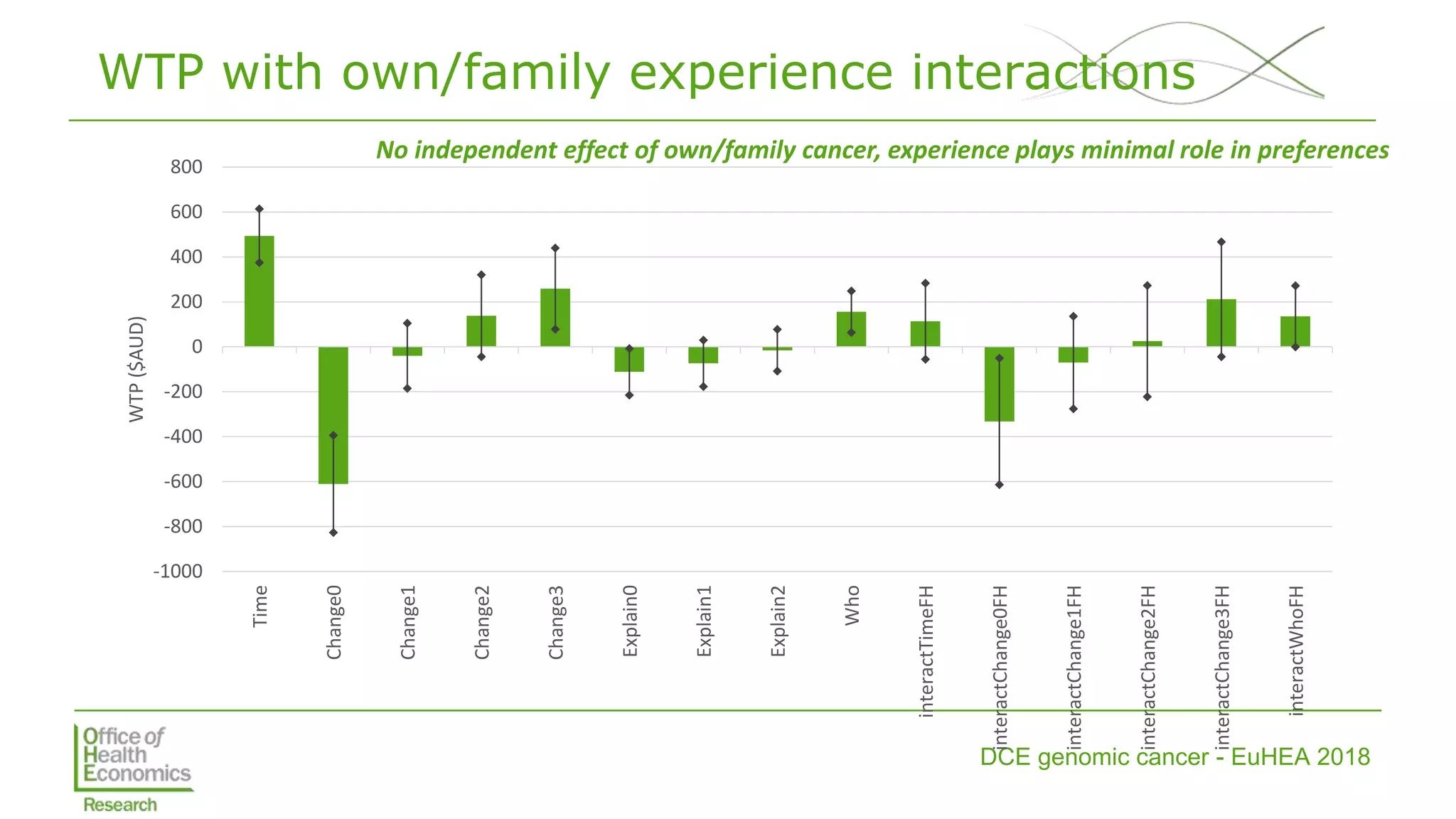
The document discusses a discrete choice experiment (DCE) aimed at understanding public preferences for genomic testing in advanced cancers, highlighting key attributes such as cost, result timeliness, and expertise of the clinician. Preliminary findings from the study indicate that cost and test efficiency are primary factors influencing preferences, with significant differences observed between general public and patients' views. Future steps include evaluating preference heterogeneity and comparing public preferences with those of actual patients undergoing genomic testing.
![DCE genomic cancer - EuHEA 2018
Objective
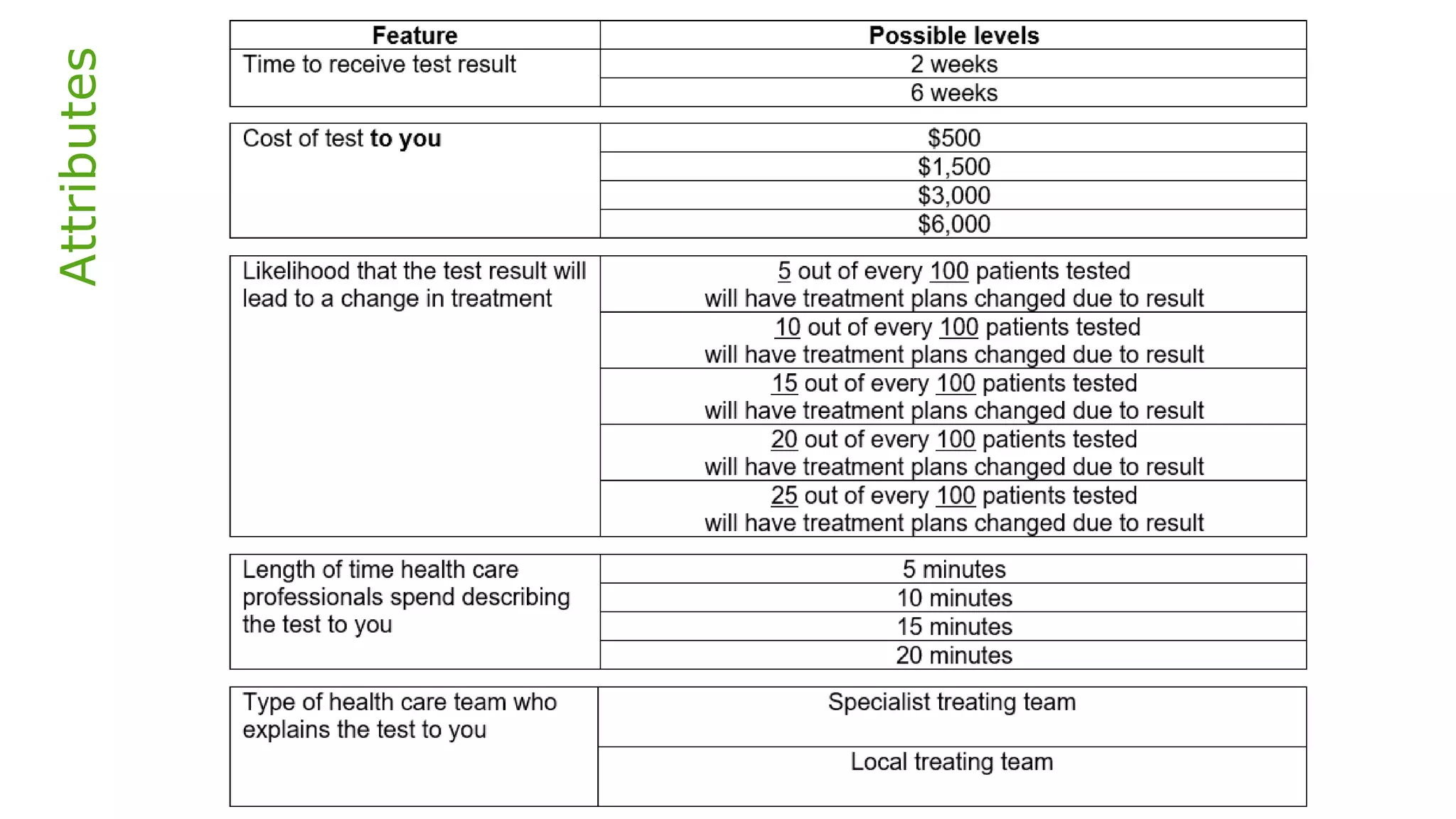
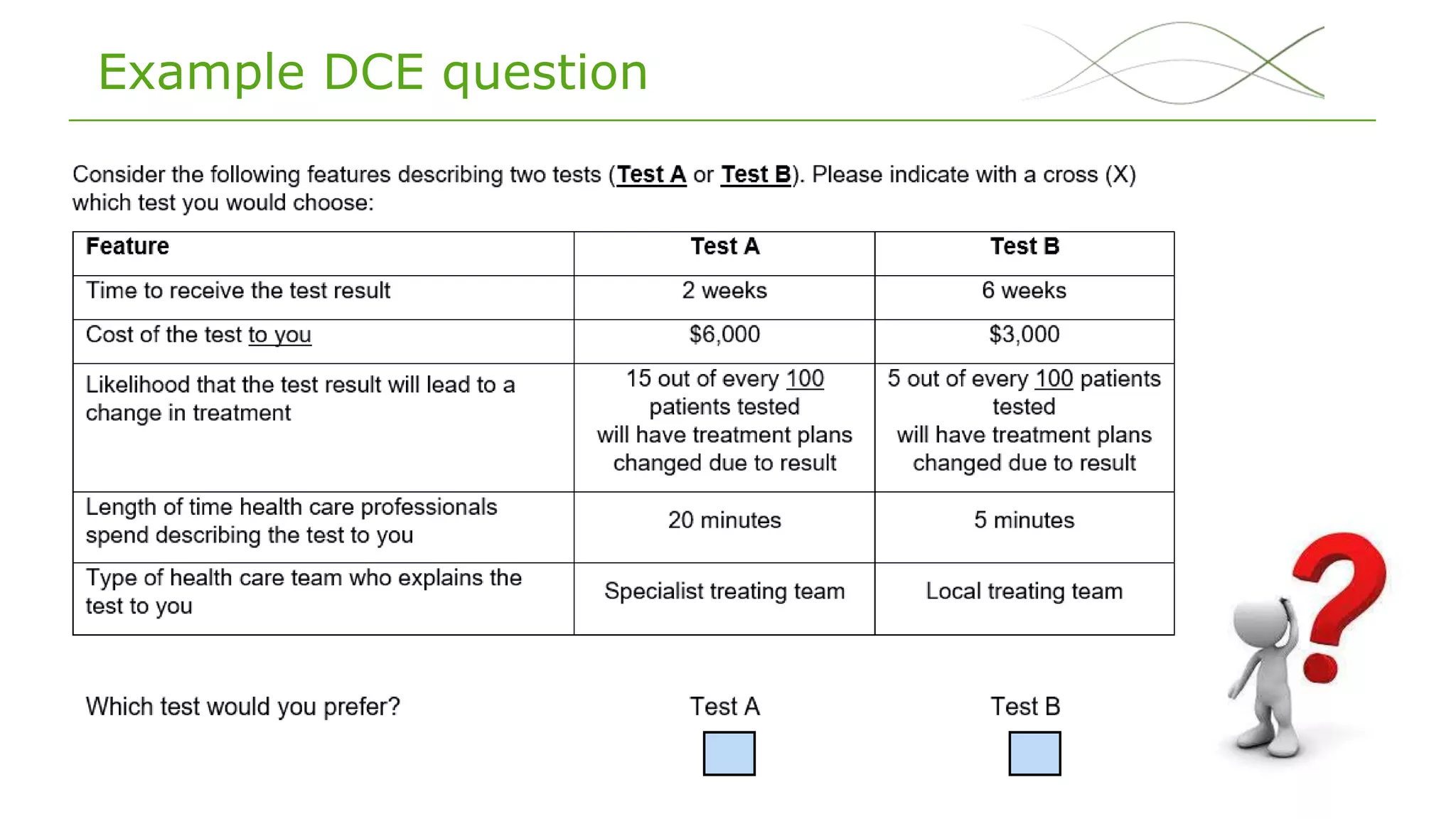
• To elicit the public’s preferences for different features of a
genomic test to sequence advanced solid cancer tumours
• [Ongoing work eliciting patient preferences]
• Relative preferences for different attributes of targeted testing
will be useful for determining the value of sequencing
approaches, and informing technology adoption and service
design decisions](https://image.slidesharecdn.com/lorgelly-thevalueoftargetedsequencinginadvancedcancer-180730093534/75/The-Value-of-Targeted-Sequencing-in-Advanced-Cancer-DCE-to-Elicit-the-Public-s-Preferences-3-2048.jpg)
![DCE genomic cancer - EuHEA 2018
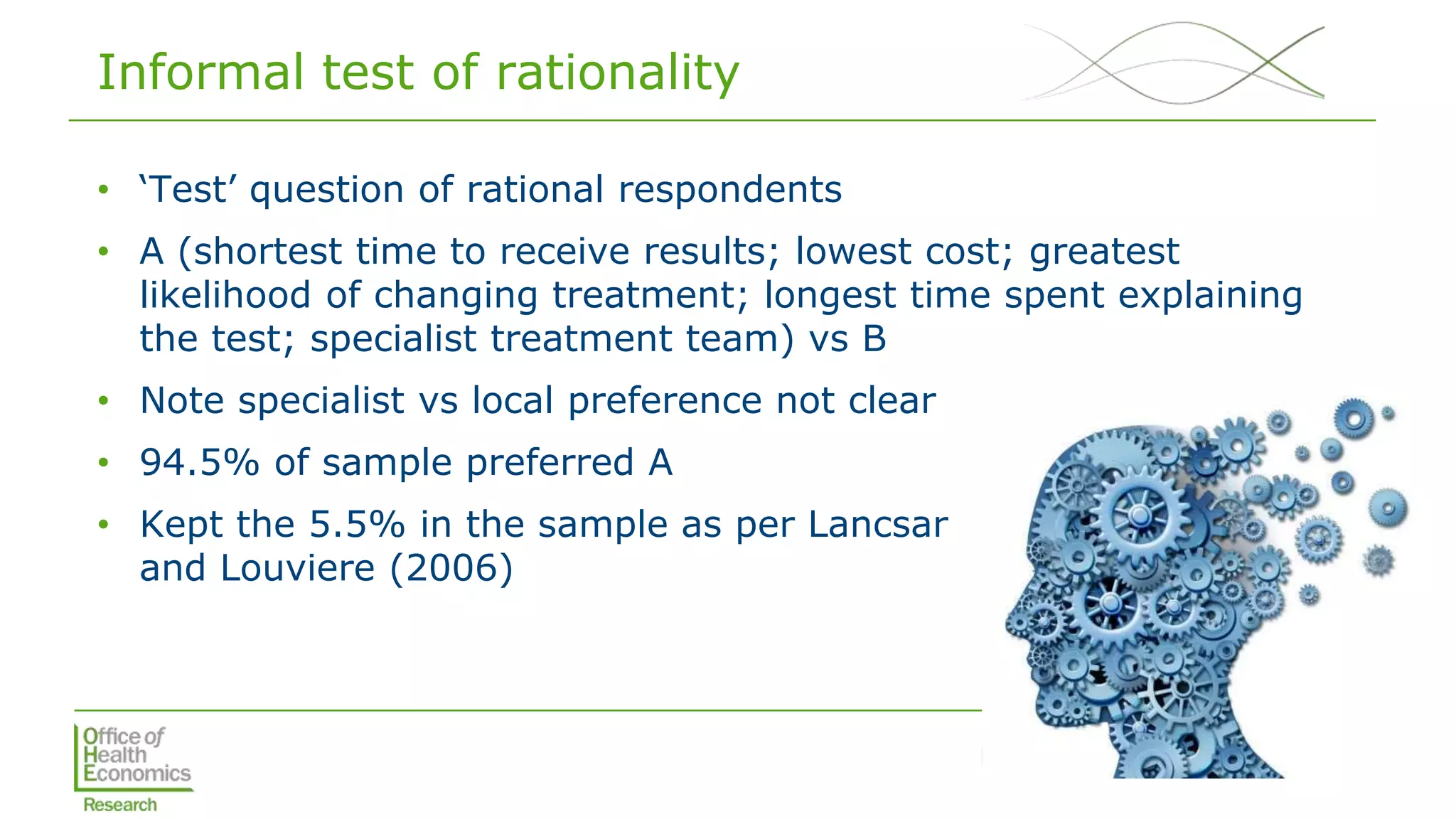
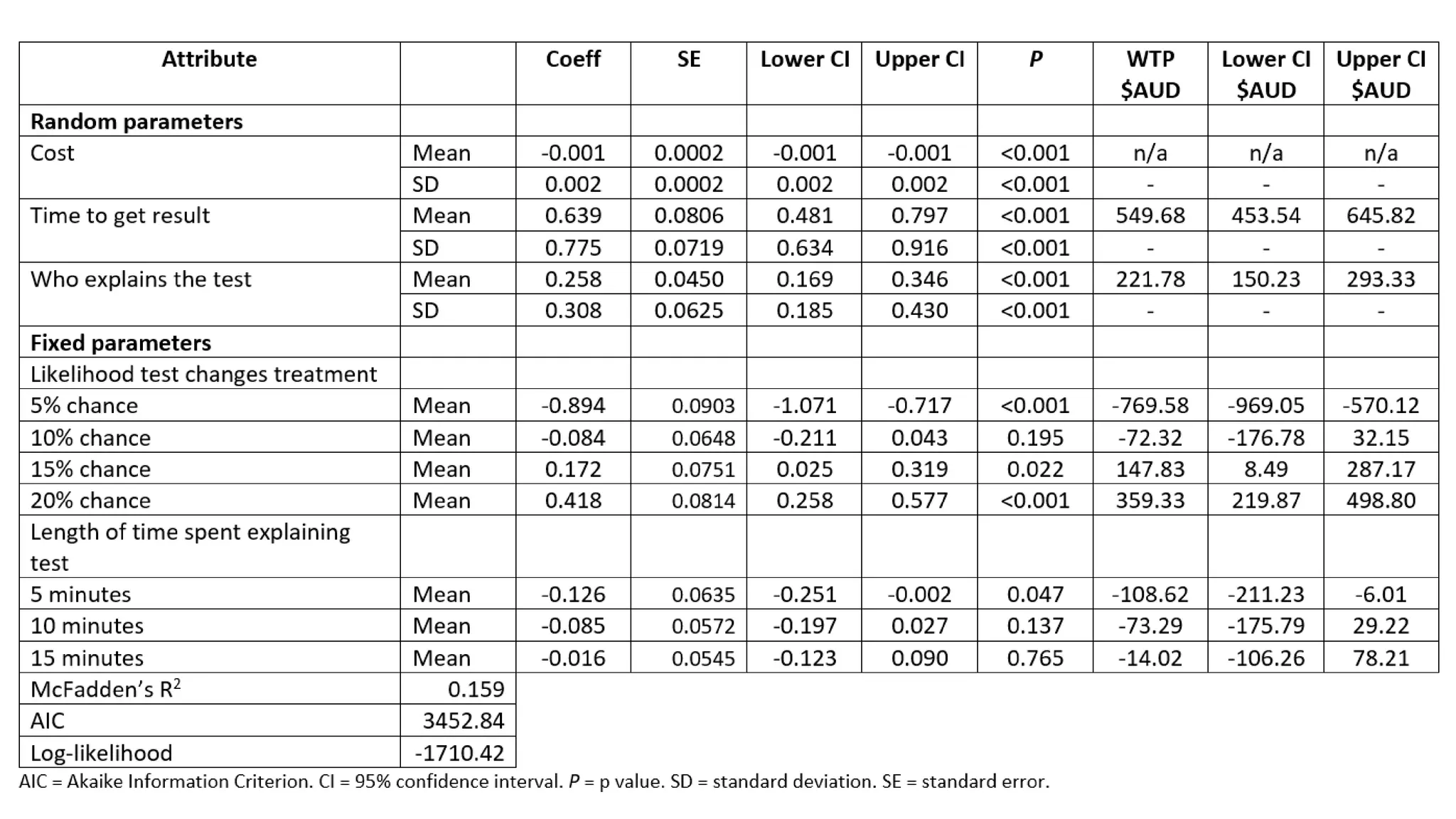
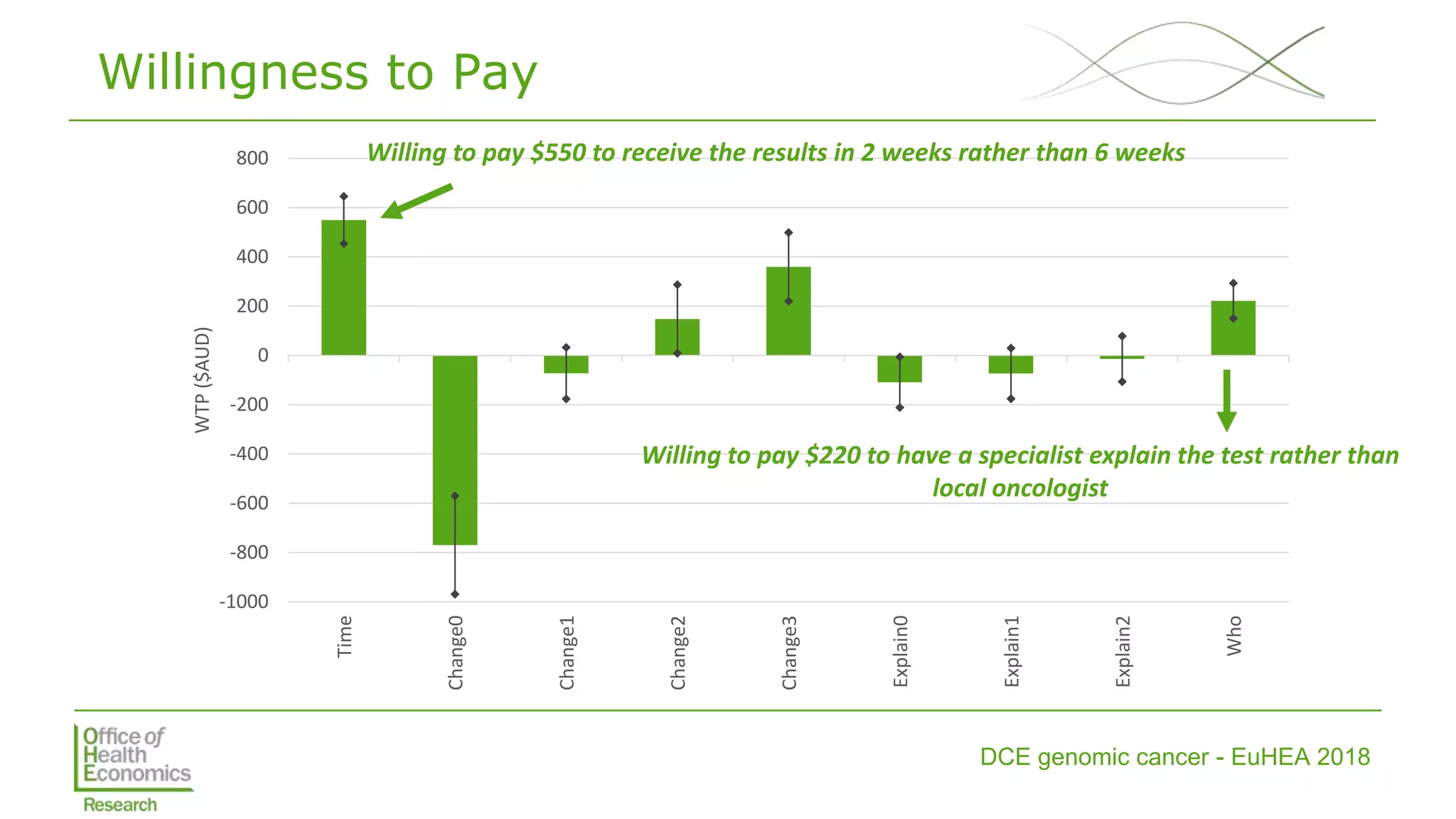
Analysis
• Mixed logit regression analysis [mixlogit]
• Iterative process to select the appropriate number of Halton draws
for the estimation process, best fit given AIC & BIC [nrep(500)]
• Next determined which parameters will be
fixed and which will be random; random
parameters those with SD that were
significant
• Adjusted the regression model to allow for
correlation between attributes
• Estimated MRS (including WTP) and tested
interactions with own/family experience](https://image.slidesharecdn.com/lorgelly-thevalueoftargetedsequencinginadvancedcancer-180730093534/75/The-Value-of-Targeted-Sequencing-in-Advanced-Cancer-DCE-to-Elicit-the-Public-s-Preferences-9-2048.jpg)
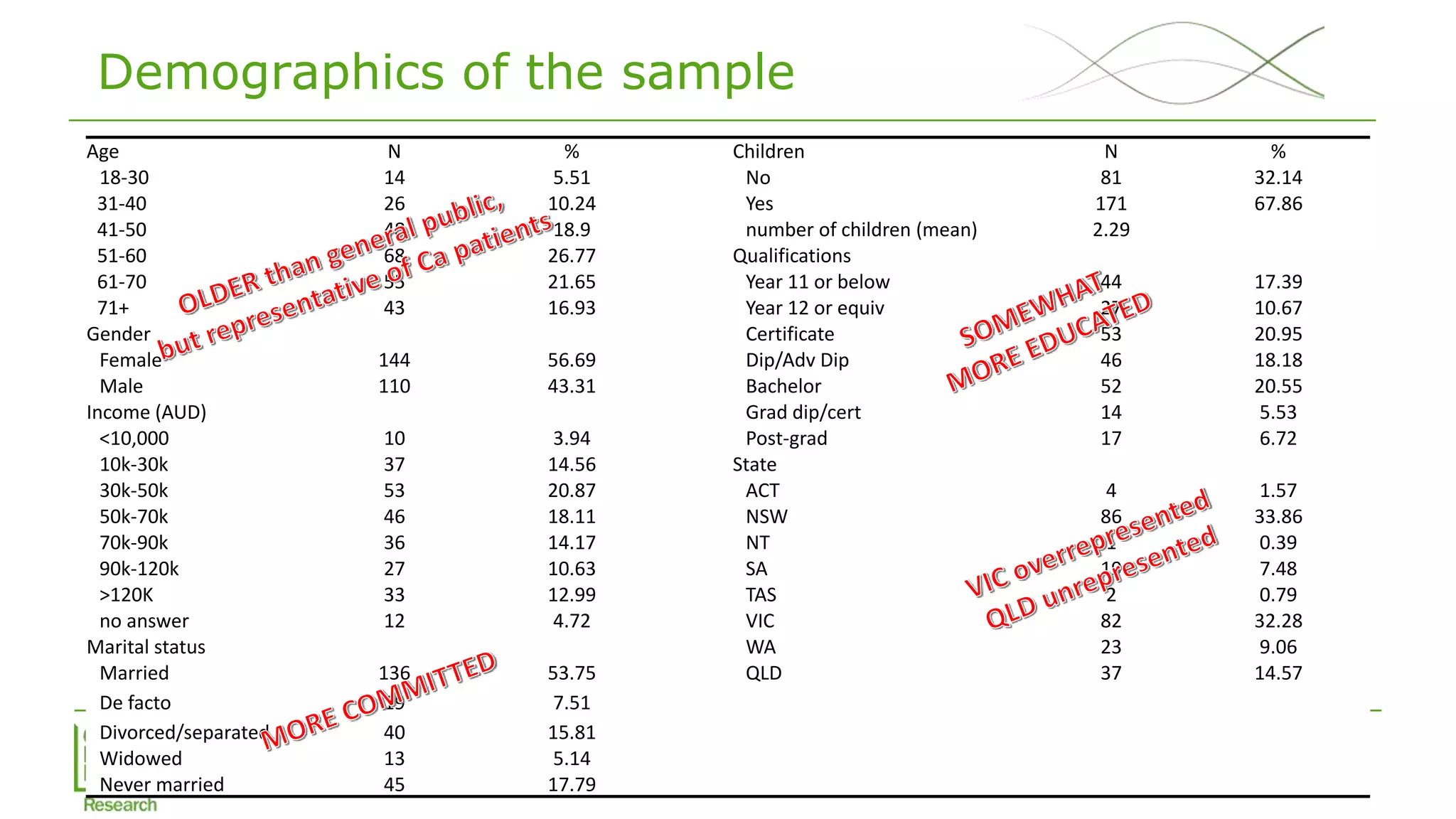
![DCE genomic cancer - EuHEA 2018
Sample
• Survey conducted Feb-March 2018
• CINT, online panel survey company
• Target sample of 125 members of general public & 125 members of
general public with own/family experience of cancer
• 33% response rate, 254 completed with 512 dropped out, 163
screened out as quotas filled [cancer experience, state, age]
• 128 (of 254) with own/family experience of cancer](https://image.slidesharecdn.com/lorgelly-thevalueoftargetedsequencinginadvancedcancer-180730093534/75/The-Value-of-Targeted-Sequencing-in-Advanced-Cancer-DCE-to-Elicit-the-Public-s-Preferences-10-2048.jpg)