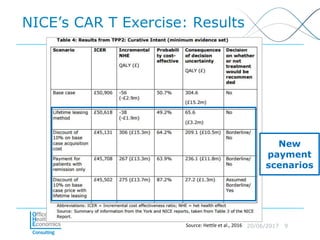
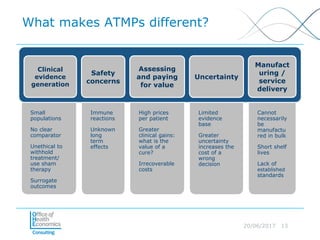
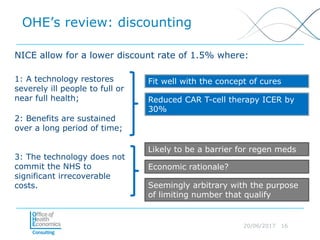
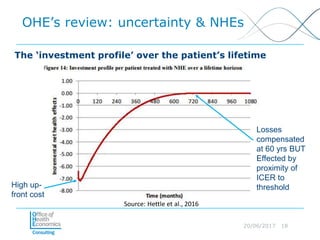
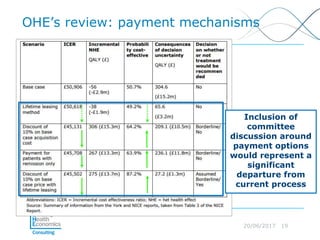
This document discusses whether current health technology assessment (HTA) methods are suitable for advanced therapy medicinal products (ATMPs) like gene and cell therapies. It summarizes a previous exercise by the National Institute for Health and Care Excellence (NICE) assessing a CAR T-cell therapy using standard methods. While NICE found existing methods applicable, the document notes the exercise did not explore all issues for ATMPs and more research is needed on topics like appropriate criteria for curative therapies and characterizing uncertainty.