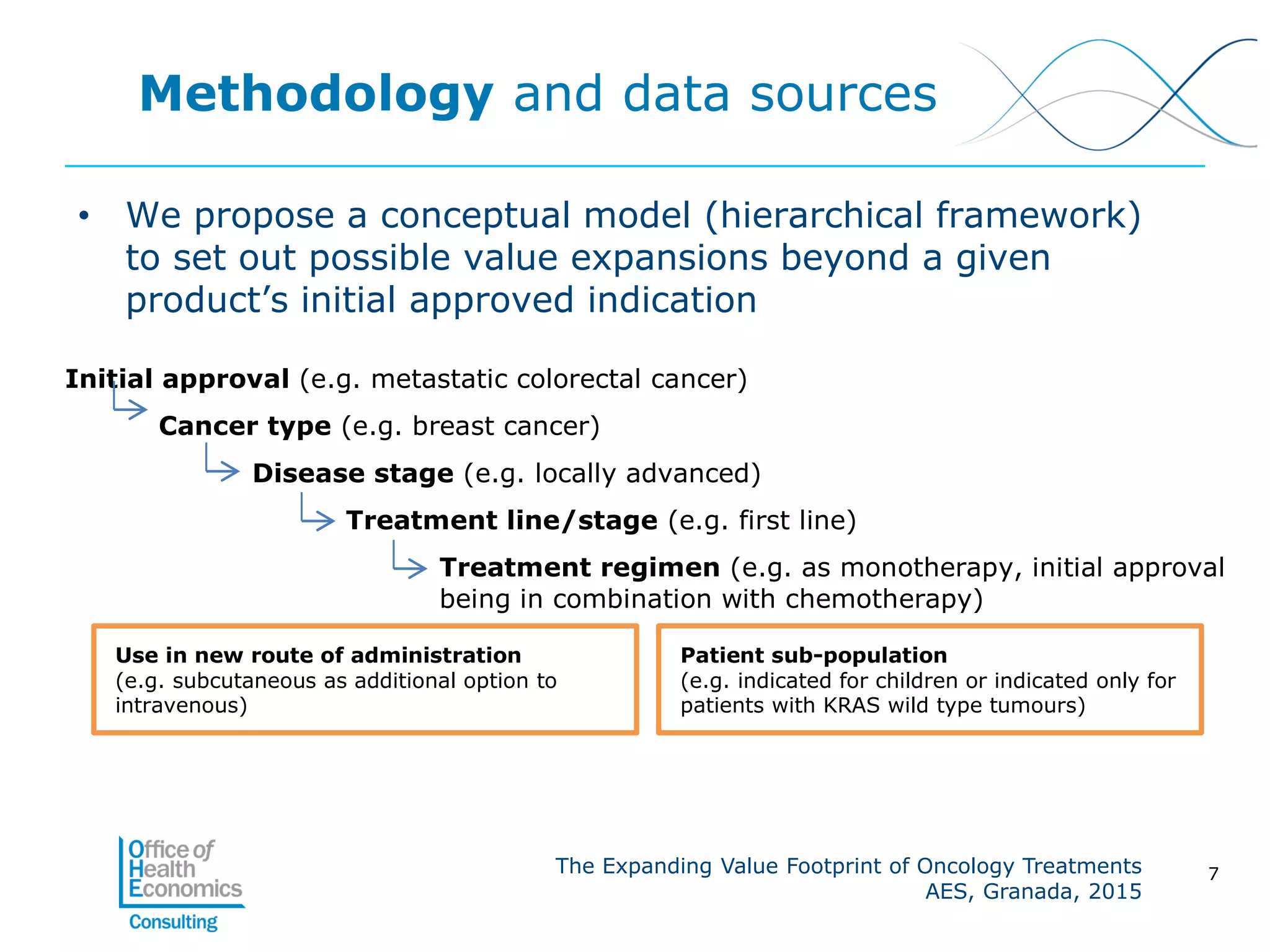
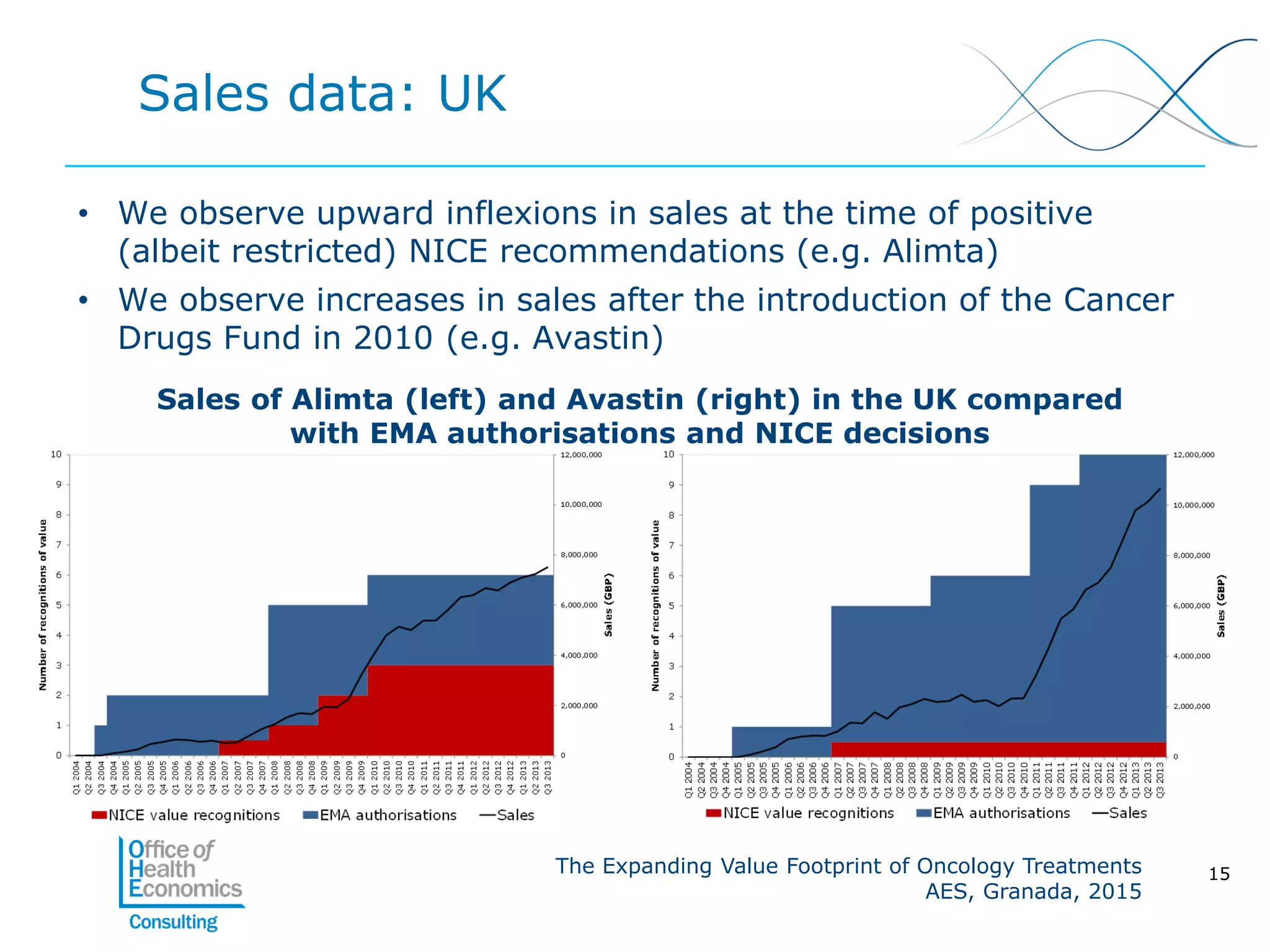
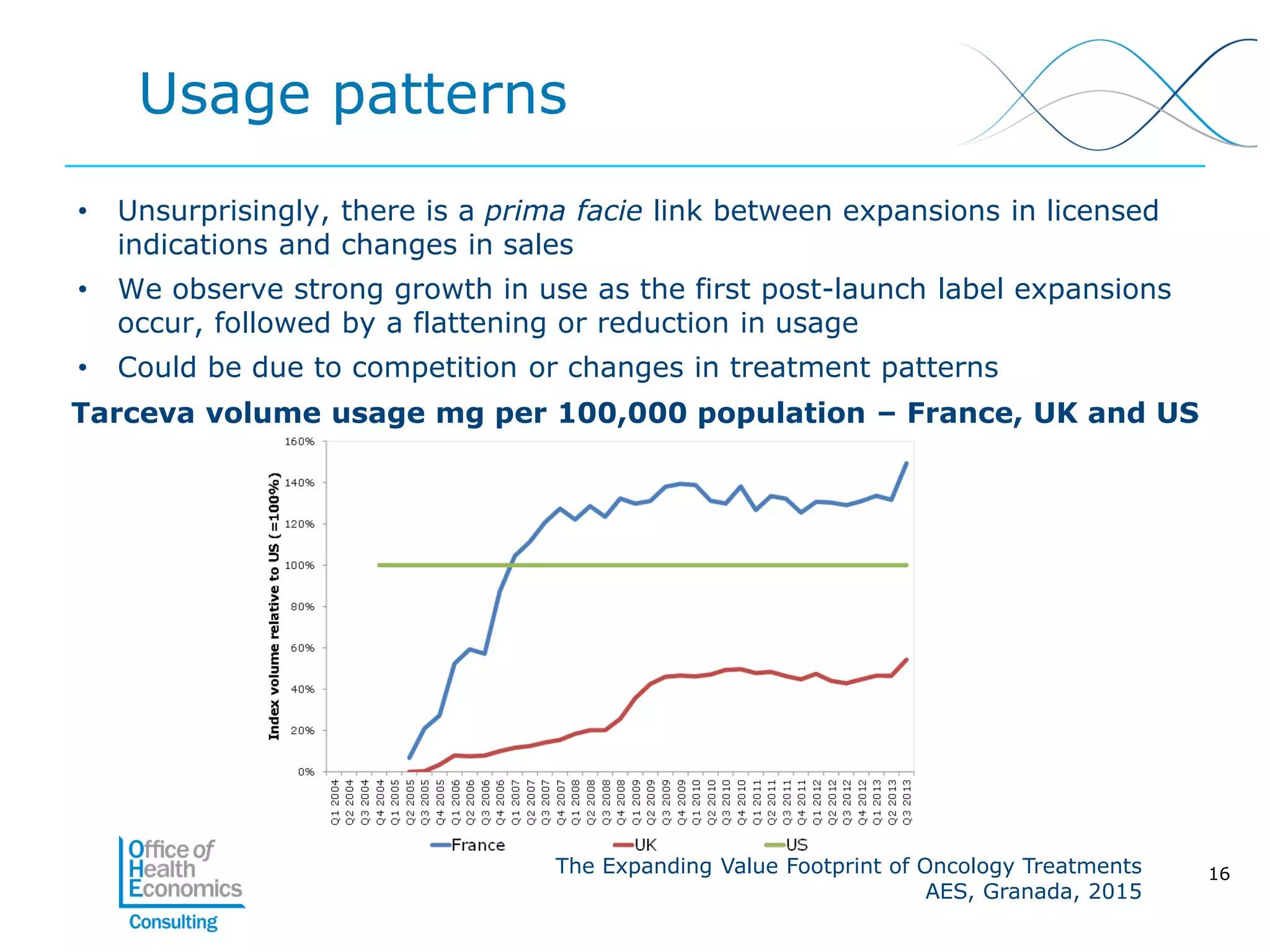
This document summarizes a study analyzing the expanding value of 10 oncology drugs approved by the EMA between 2003-2005. It finds that 7 of the 10 drugs gained additional approved indications and value expansions over time. The study tracks these expansions and analyzes data from the EMA, NICE, HAS, Aetna and IMS on prices, volumes and sales in the UK, France and US. Key conclusions are that health systems should consider a drug's evolving value over its lifecycle, and HTA processes could better incorporate indication expansions and flexibility in pricing for oncology drugs.