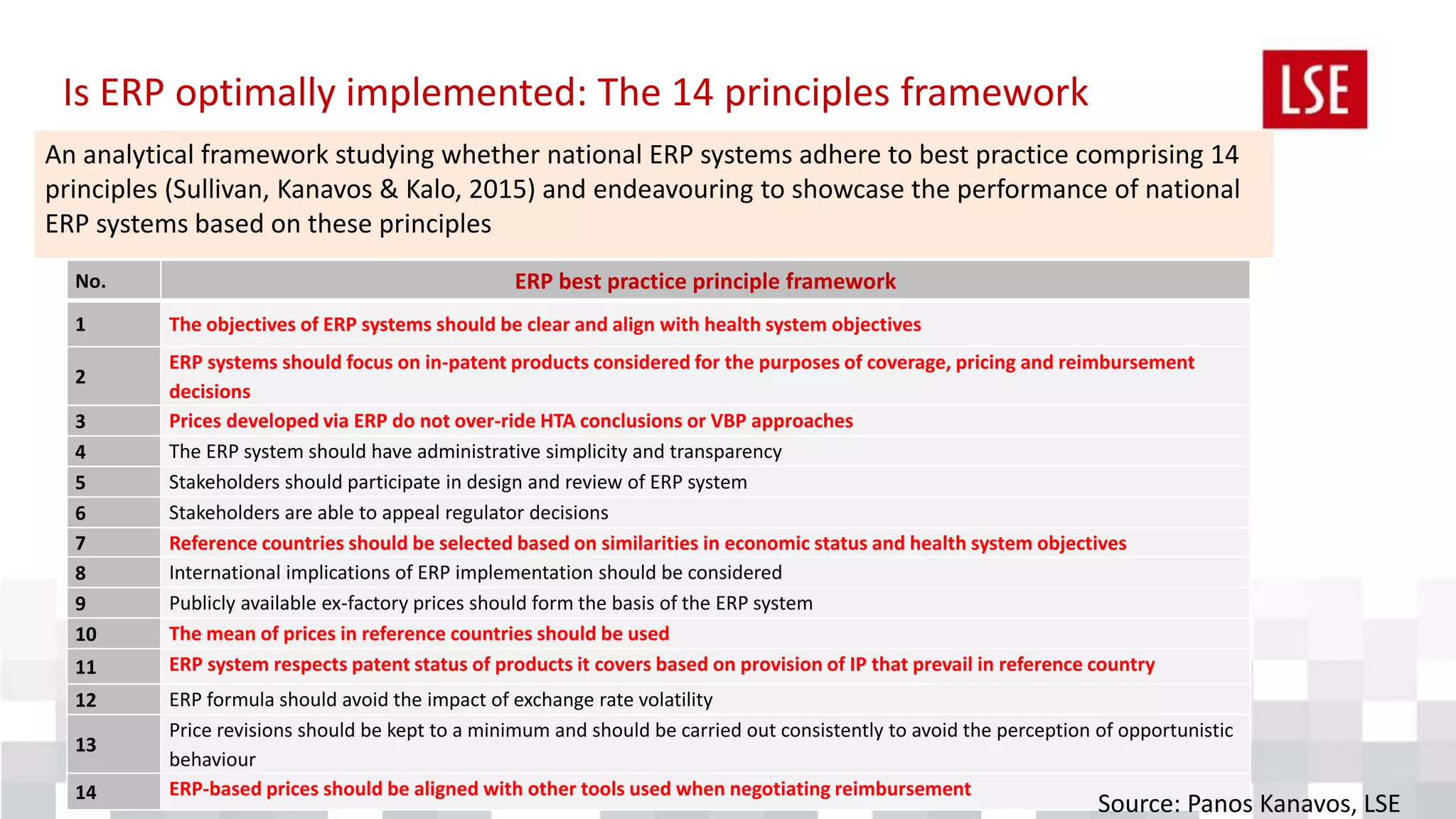
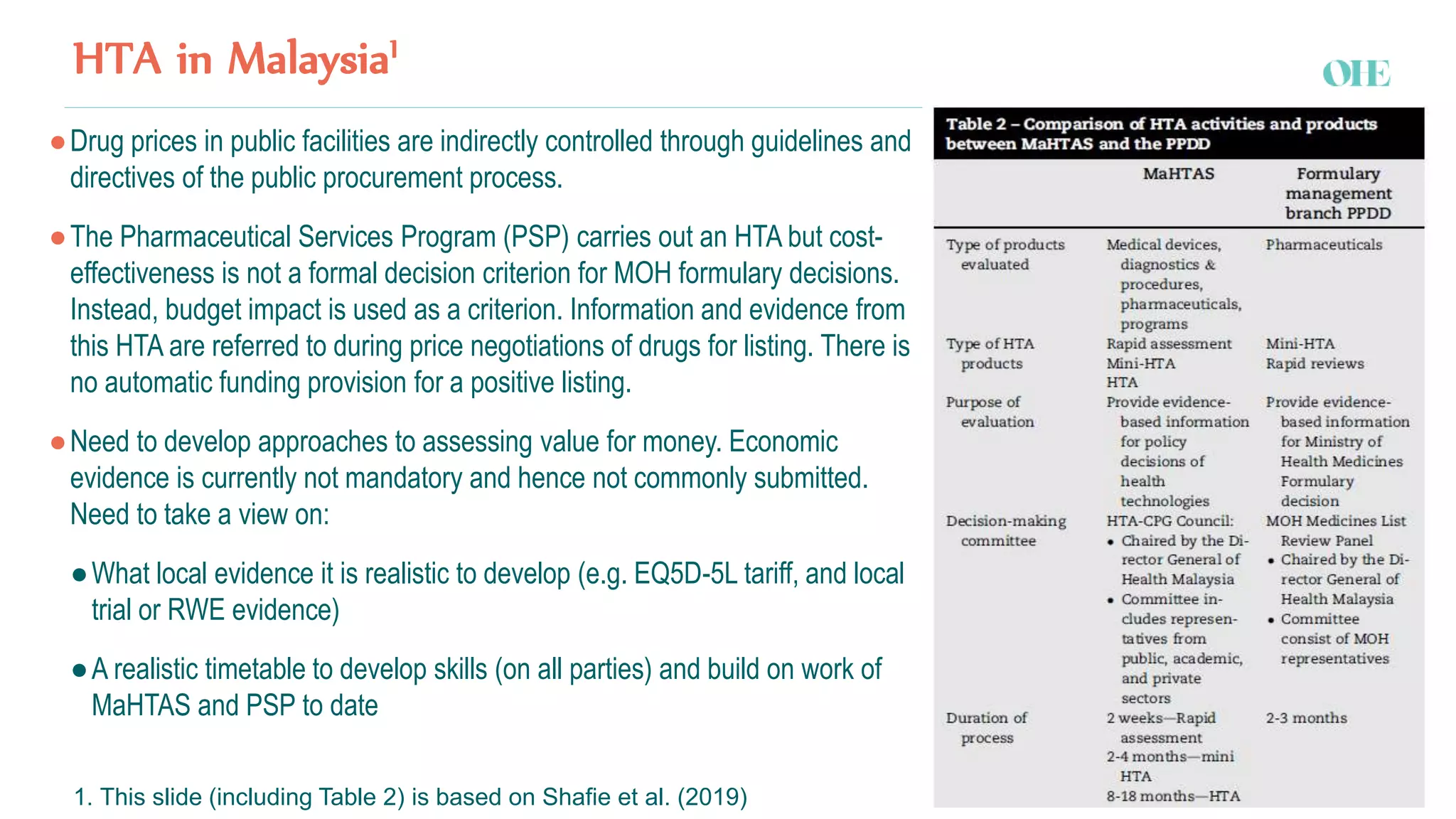
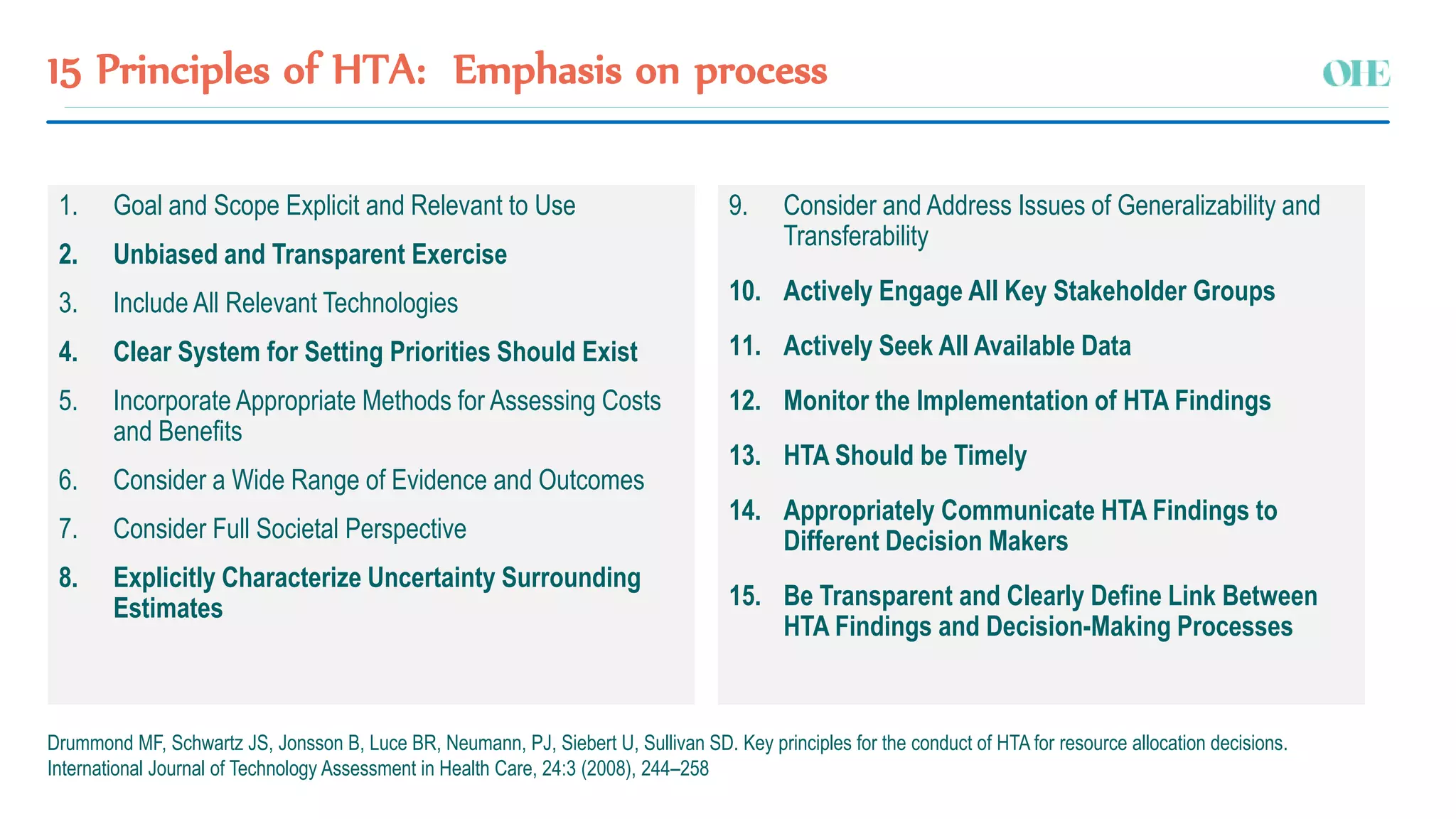
The document discusses the challenges and strategies for regulating pharmaceutical prices in Malaysia, emphasizing the rationale for intervention. It highlights the importance of differential pricing, cost-effectiveness analysis, and the role of health technology assessment (HTA) in supporting universal health coverage. The conclusions propose a shift towards value-based pricing while questioning the efficiency of external reference pricing and price control mechanisms in both out-of-pocket and off-patent markets.