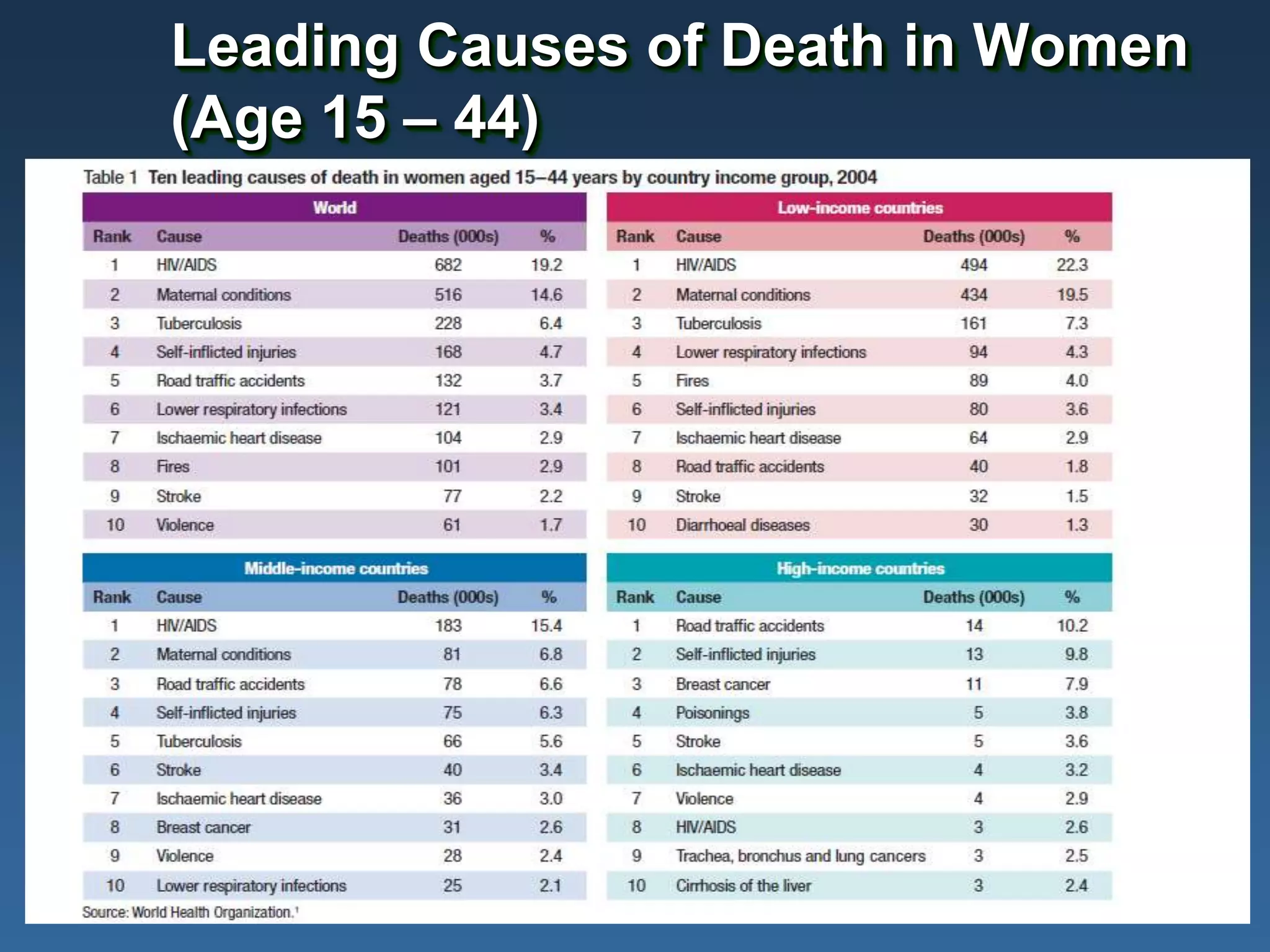
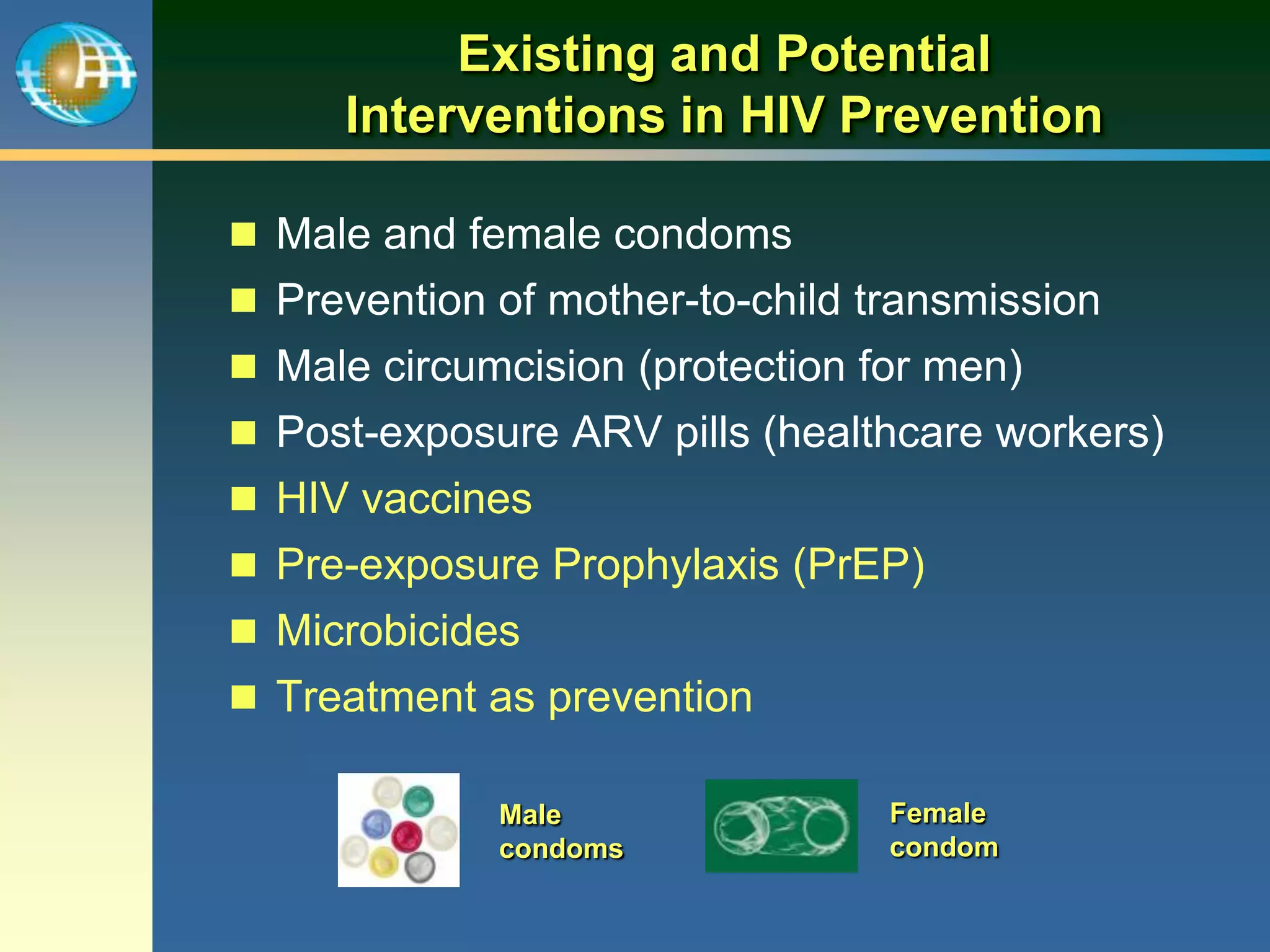
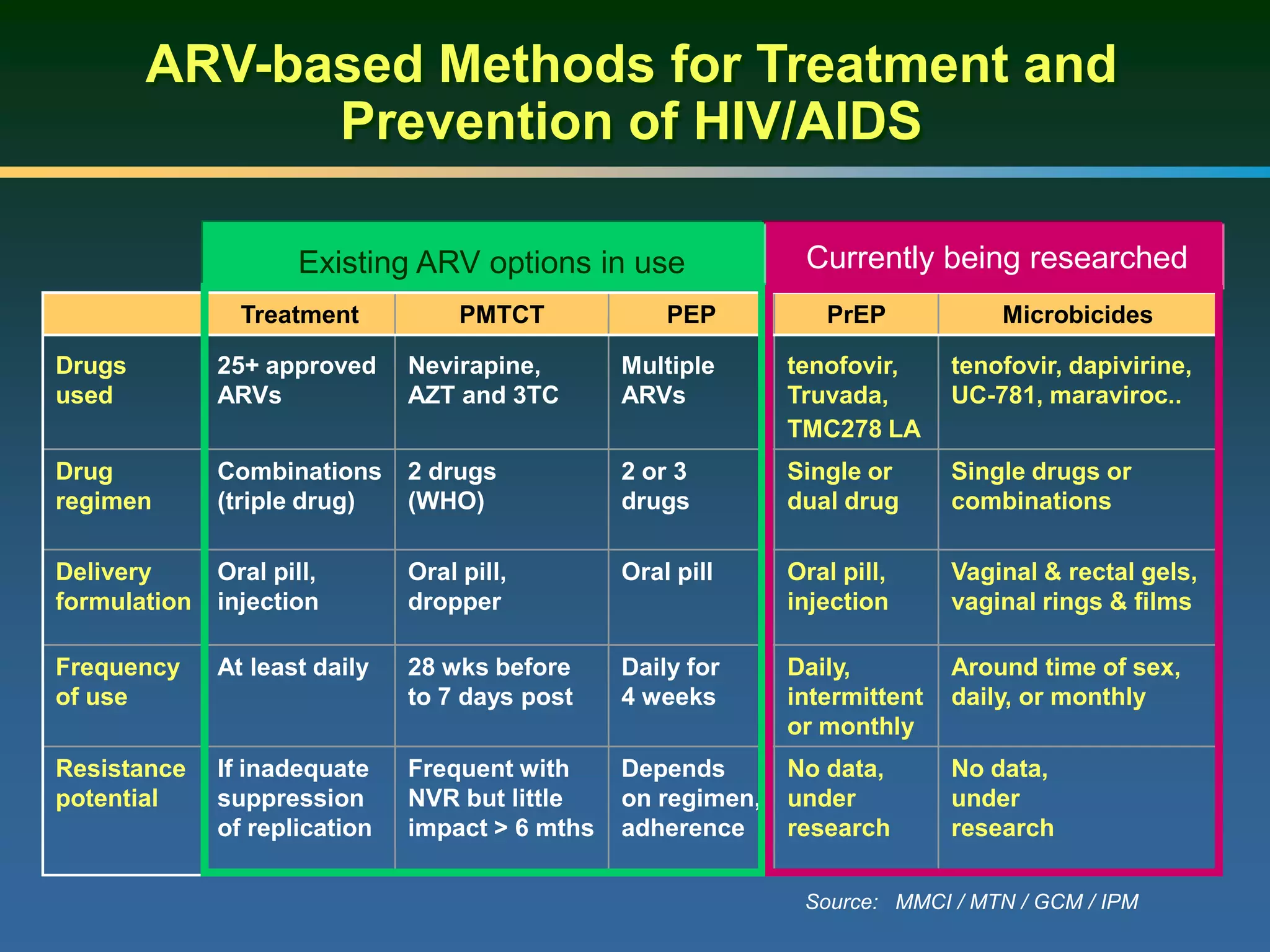
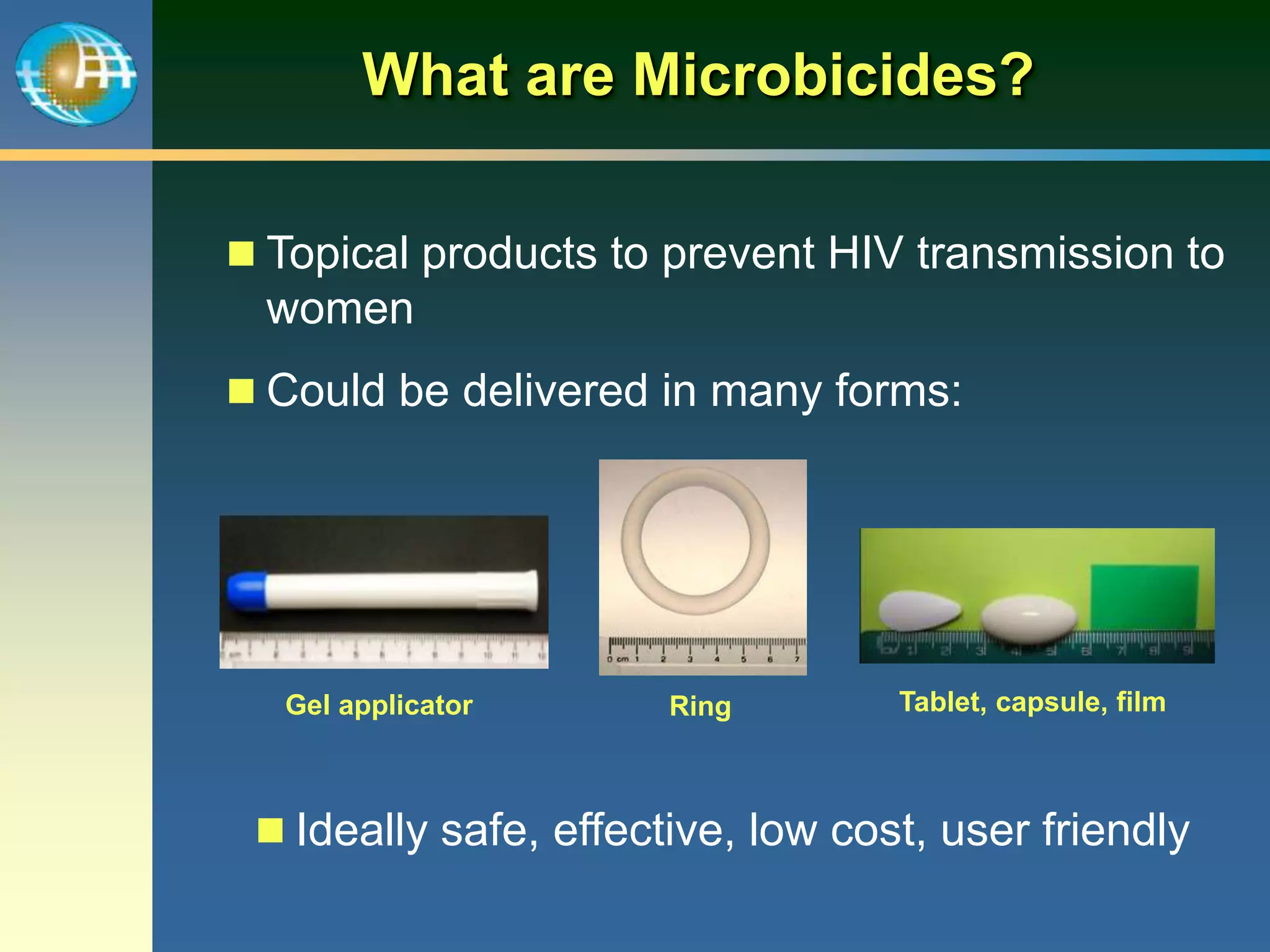
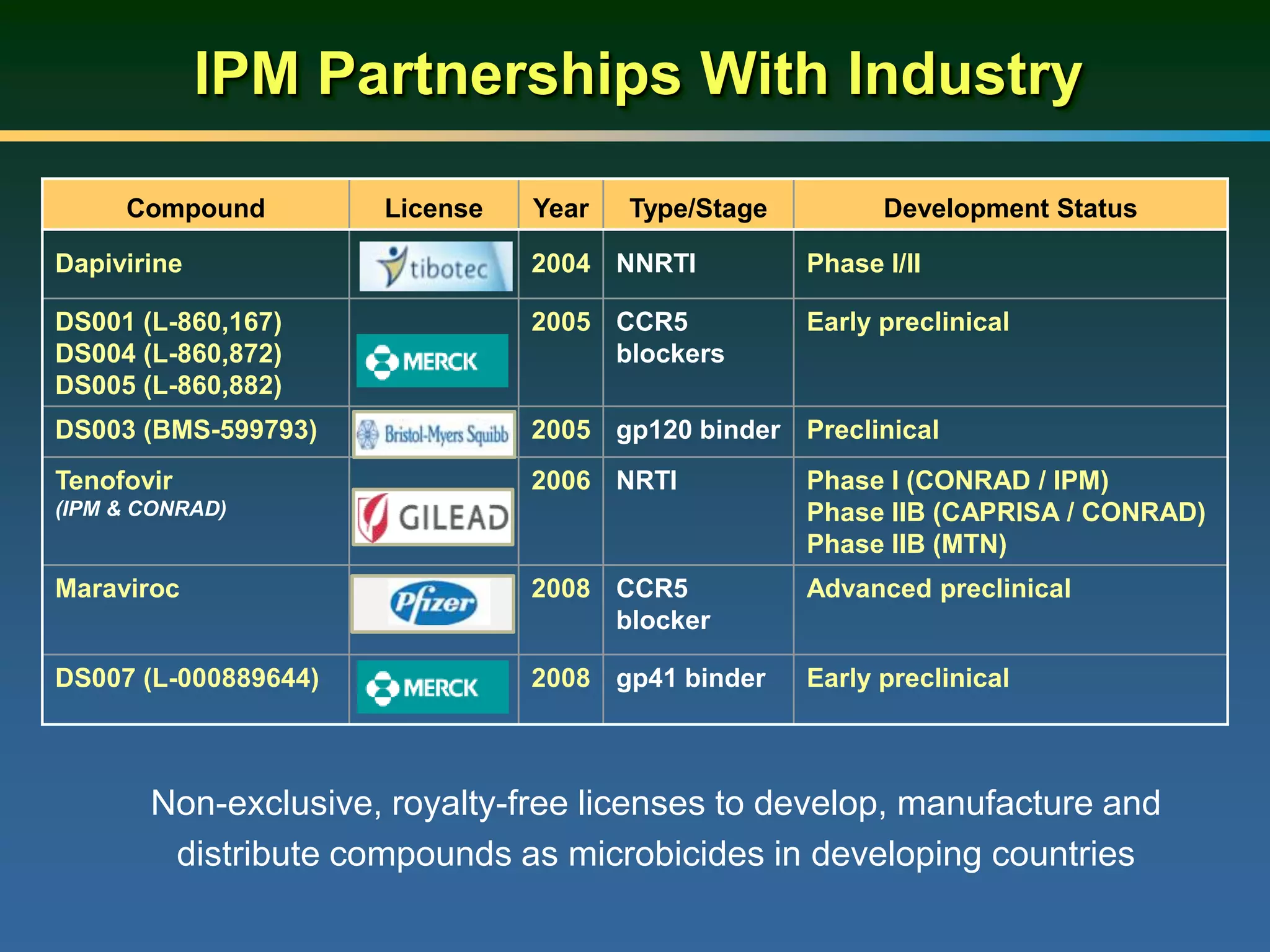
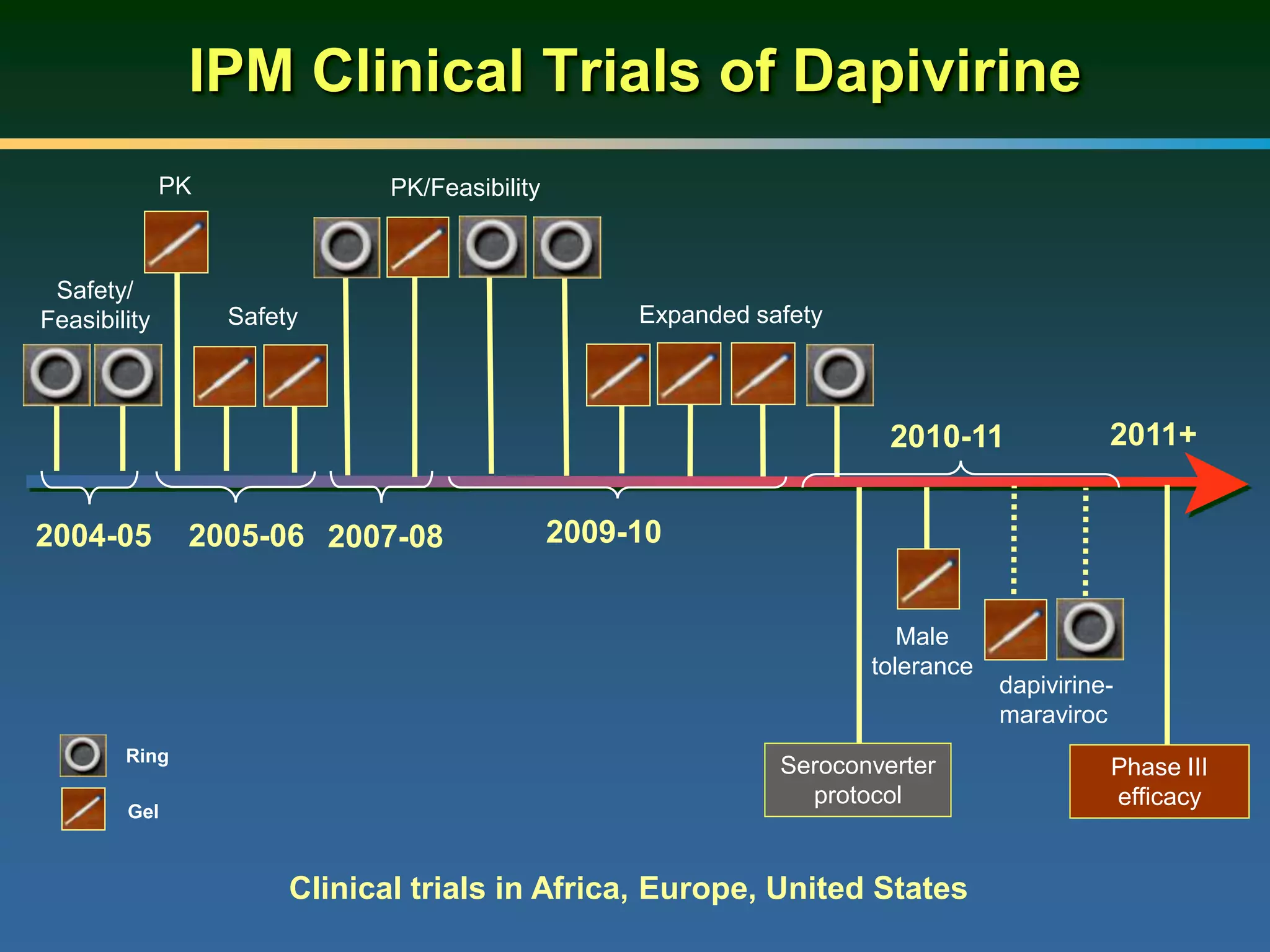
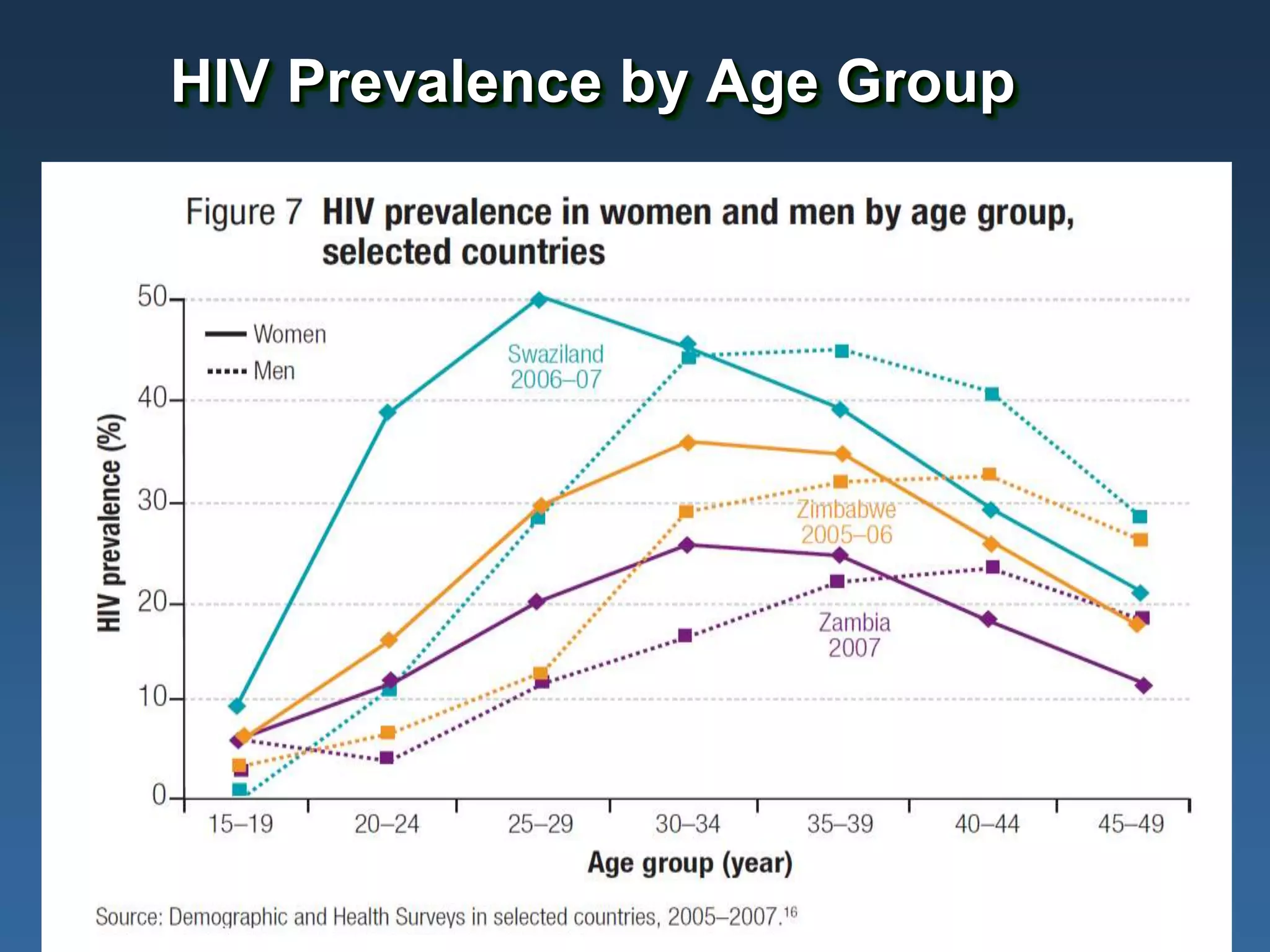
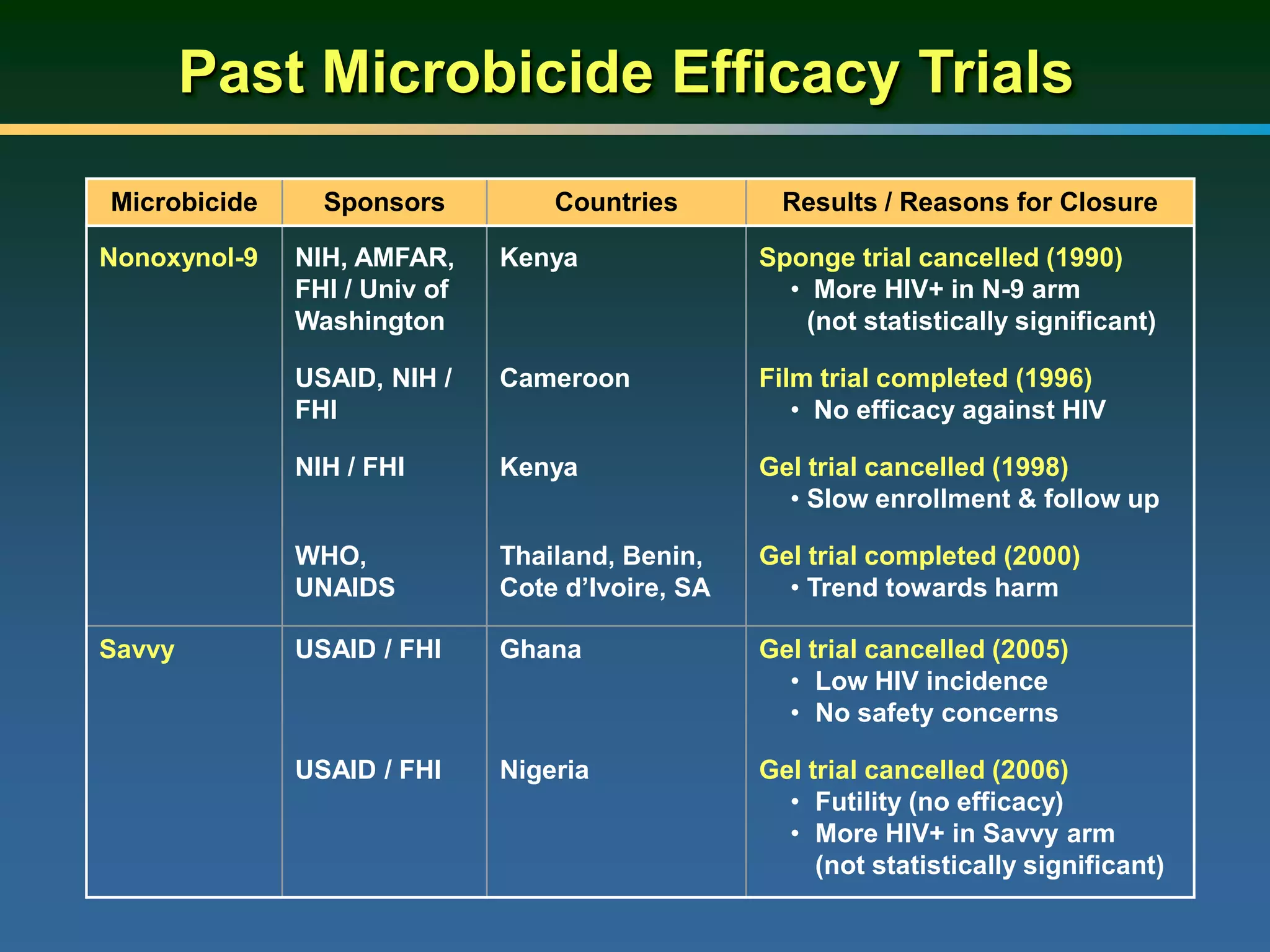
This document summarizes information presented at a consultation on new prevention technologies (NPTs) for HIV. It discusses:
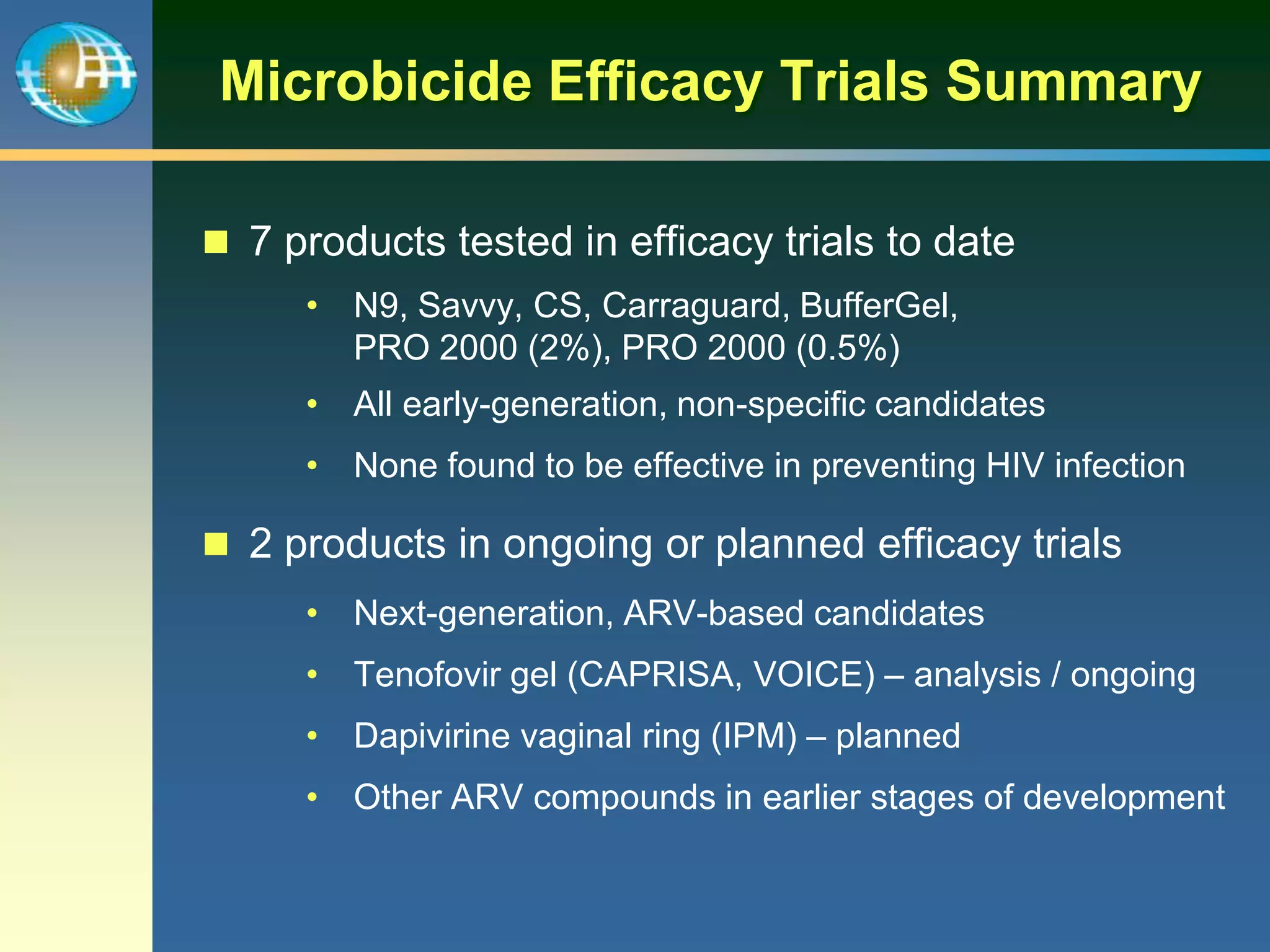
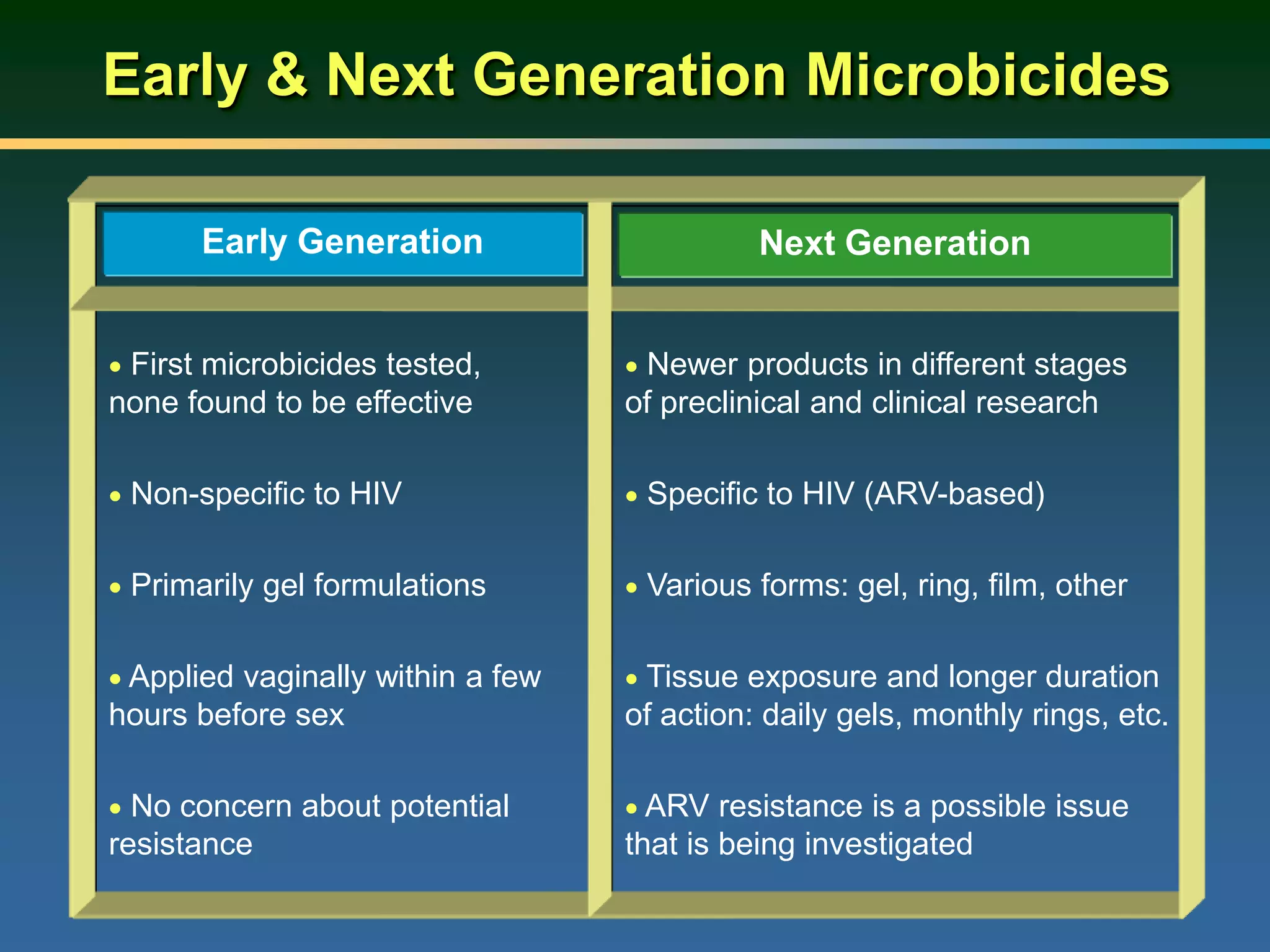
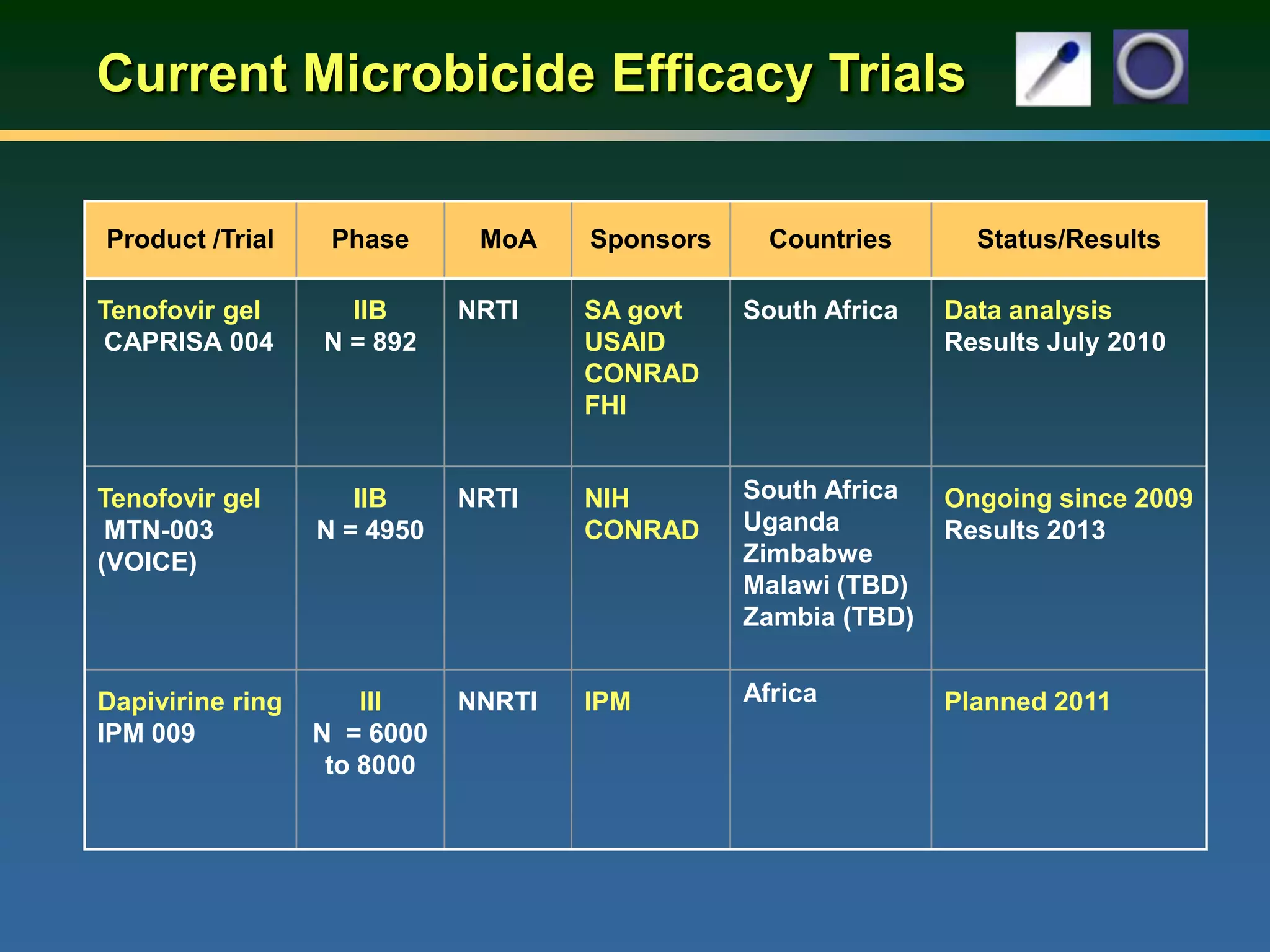
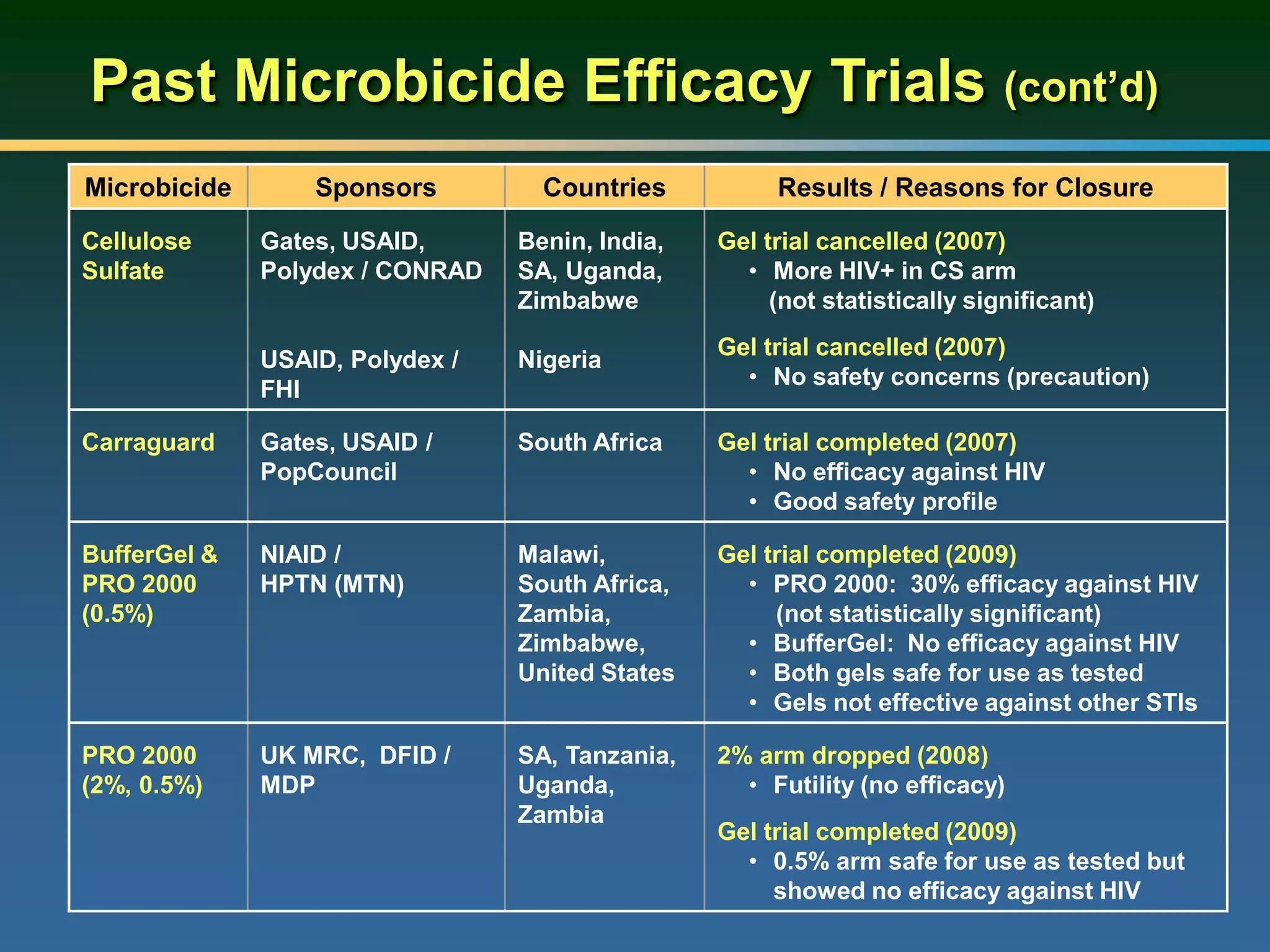
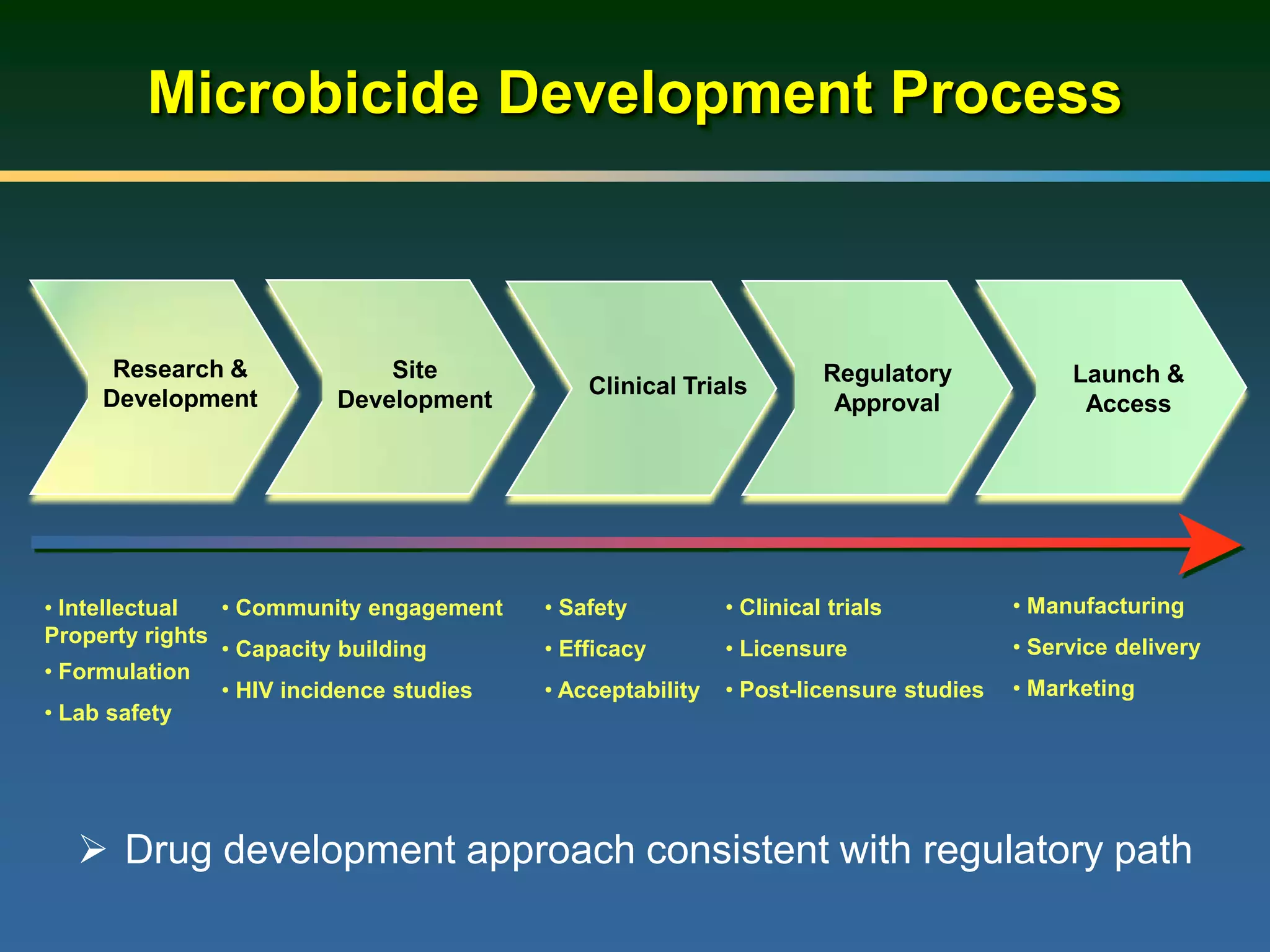
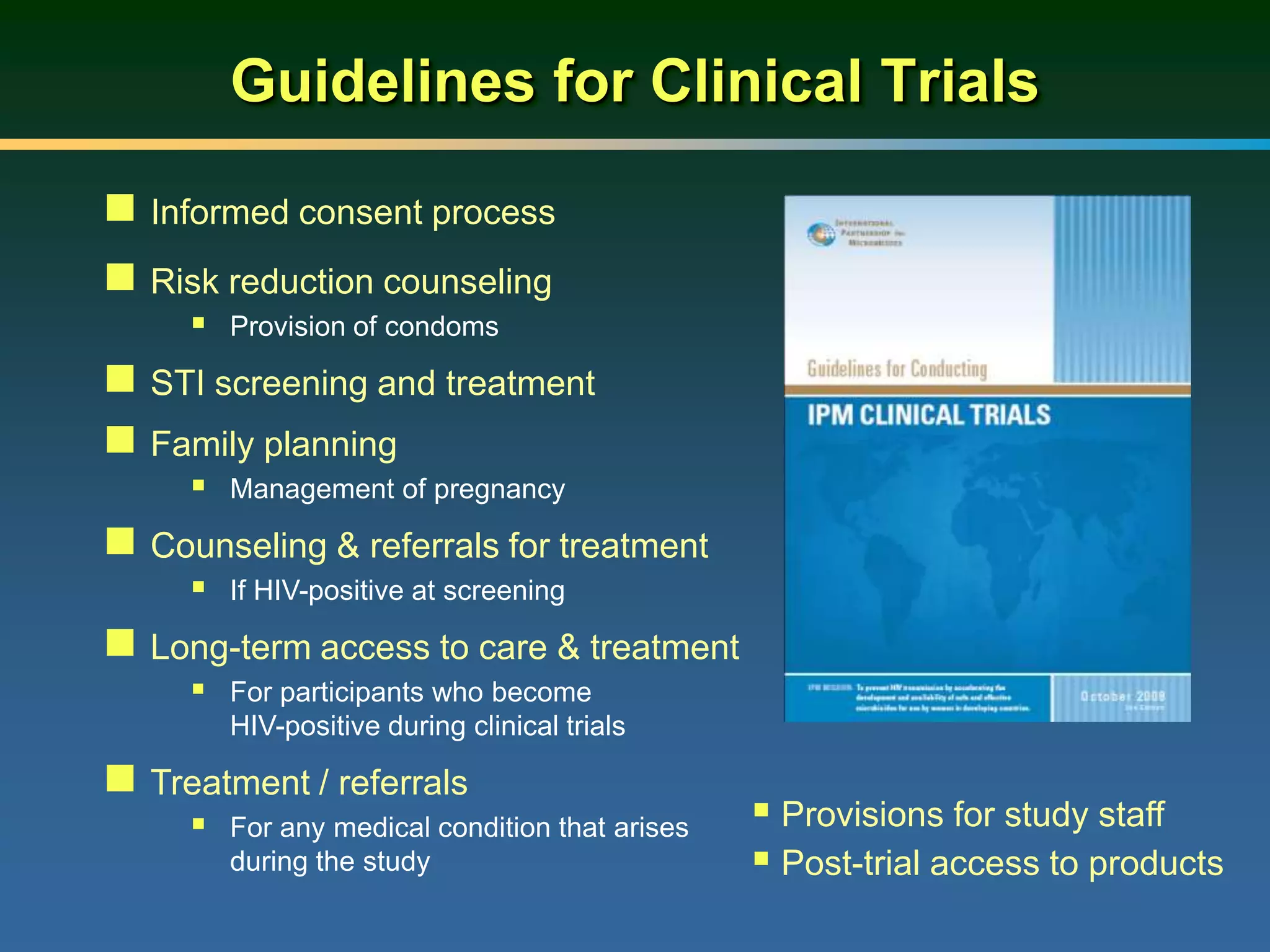
1) Existing HIV prevention options like condoms and their limitations for women. 2) Potential new options in development like microbicides, pre-exposure prophylaxis, and treatment as prevention. 3) Lessons learned from past microbicide trials that showed no efficacy. 4) Current microbicide trials testing tenofovir gel and dapivirine ring. 5) The need for women to have more prevention options they can control.