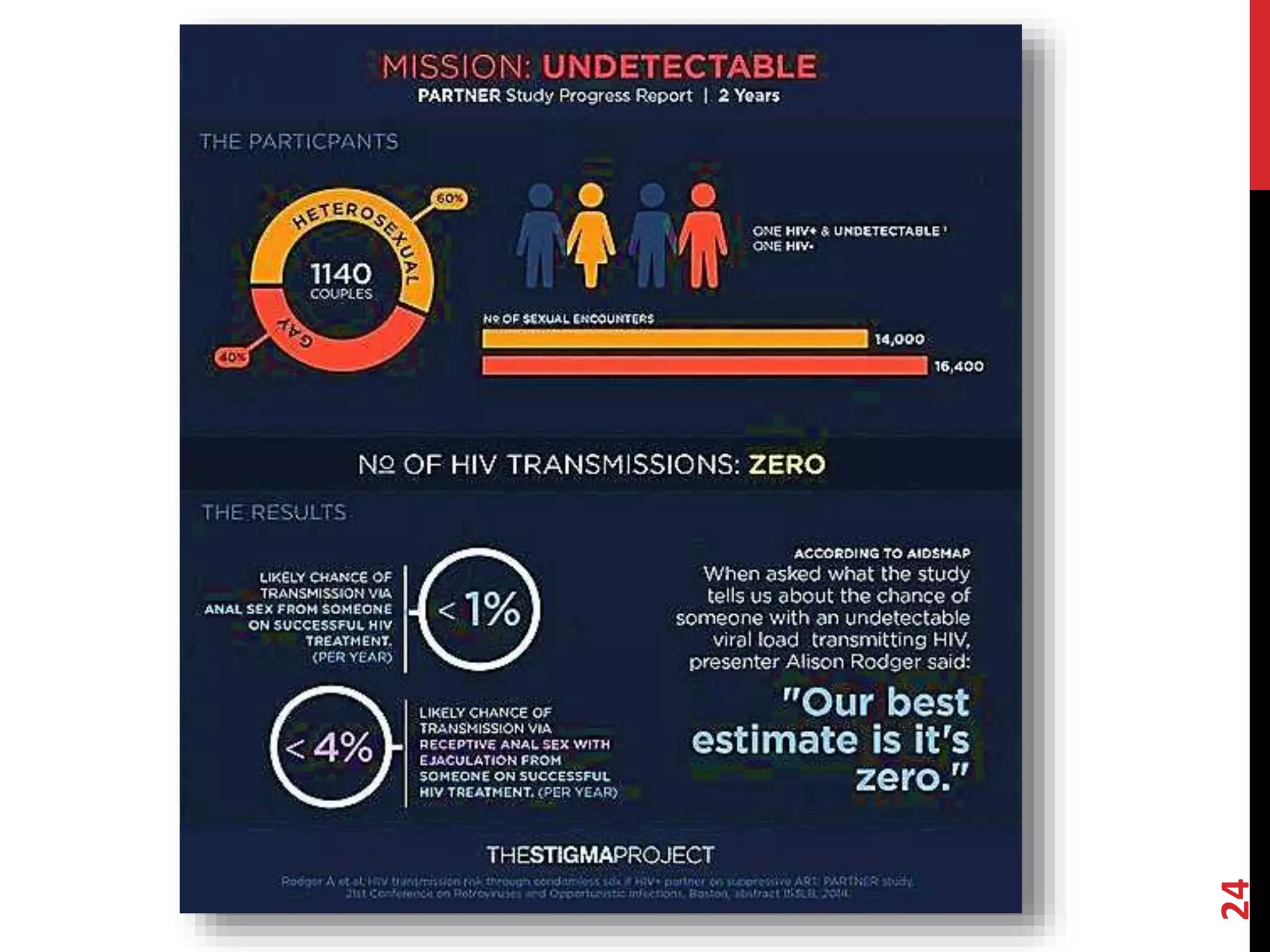
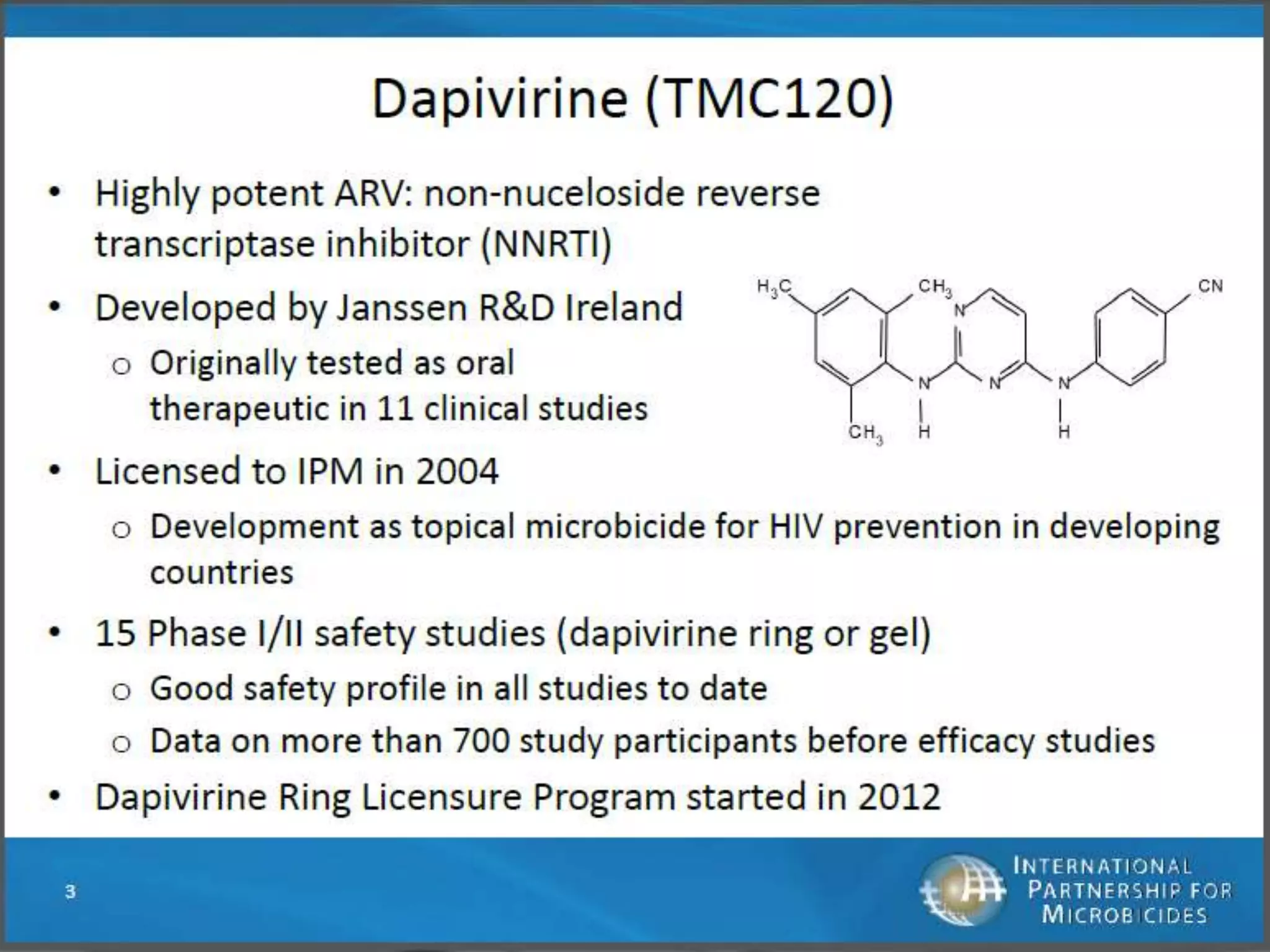
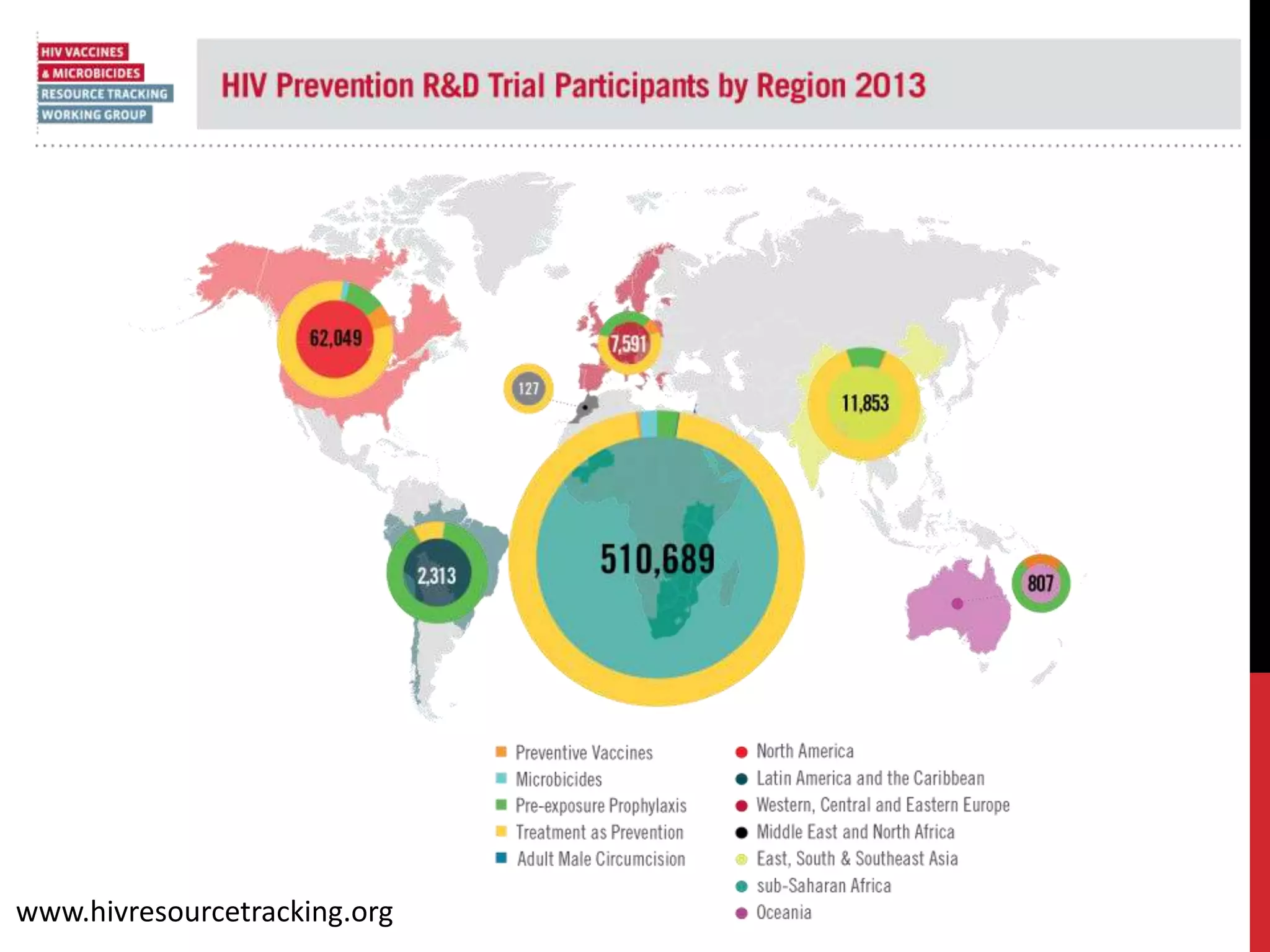
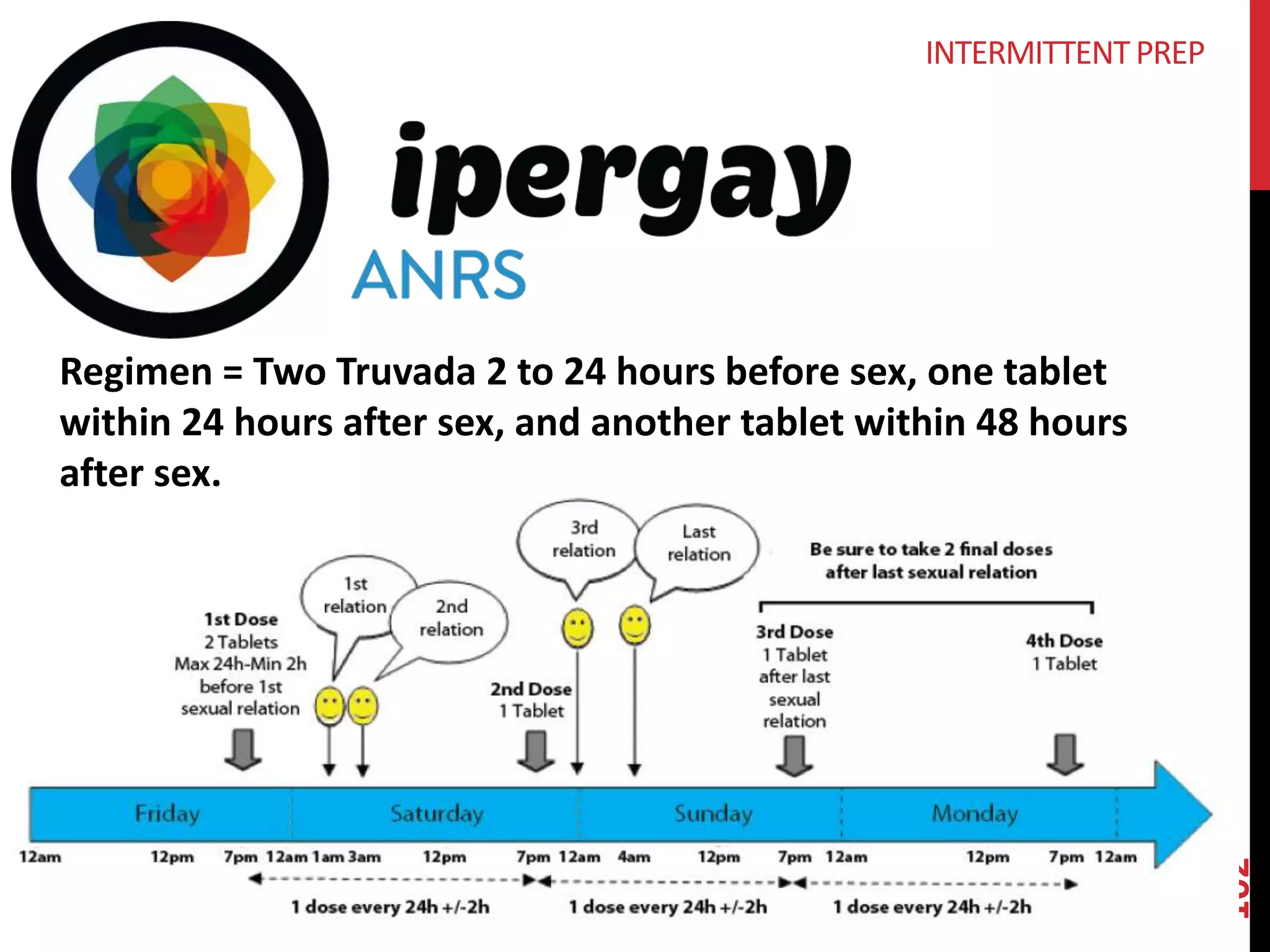
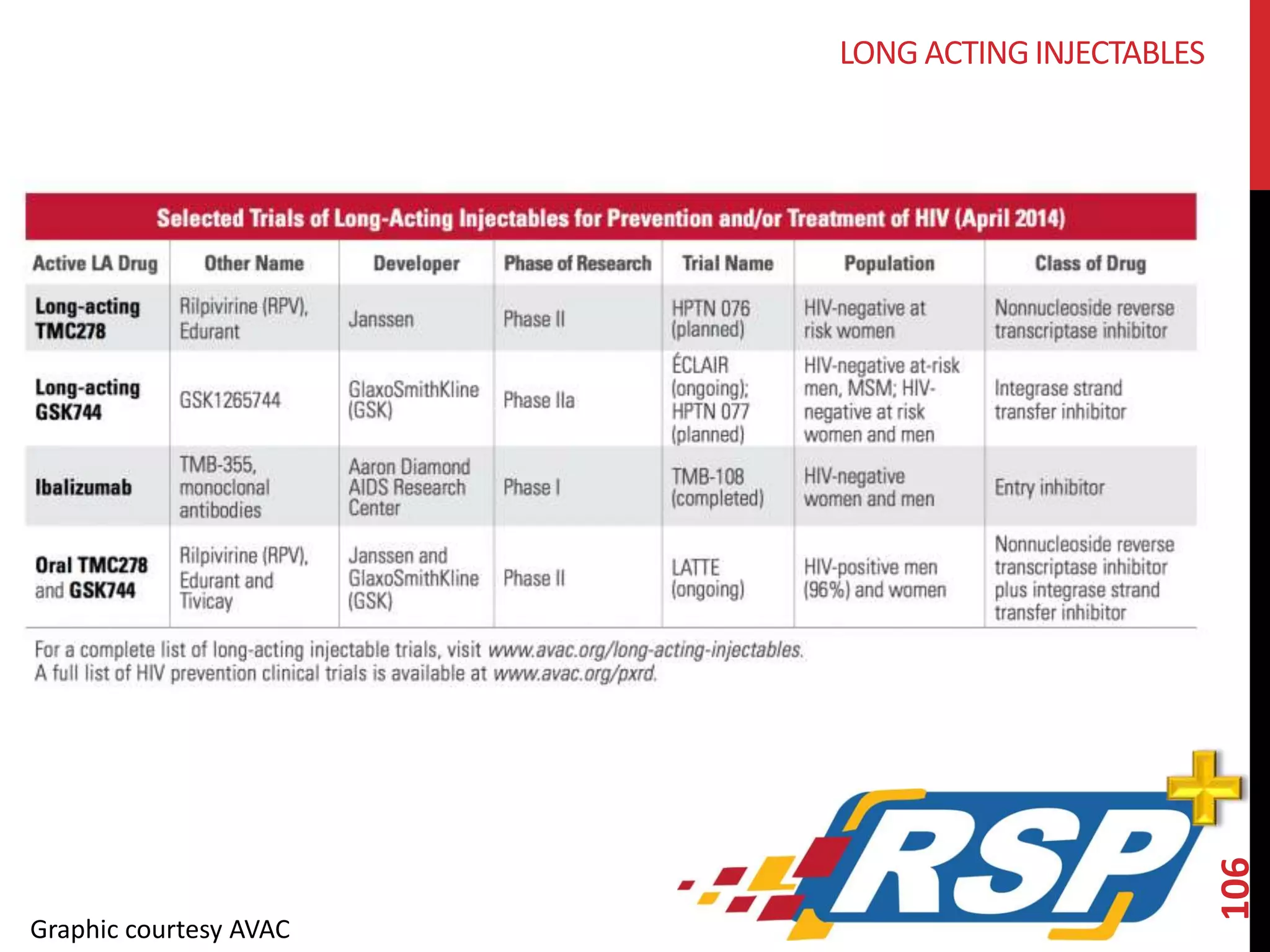
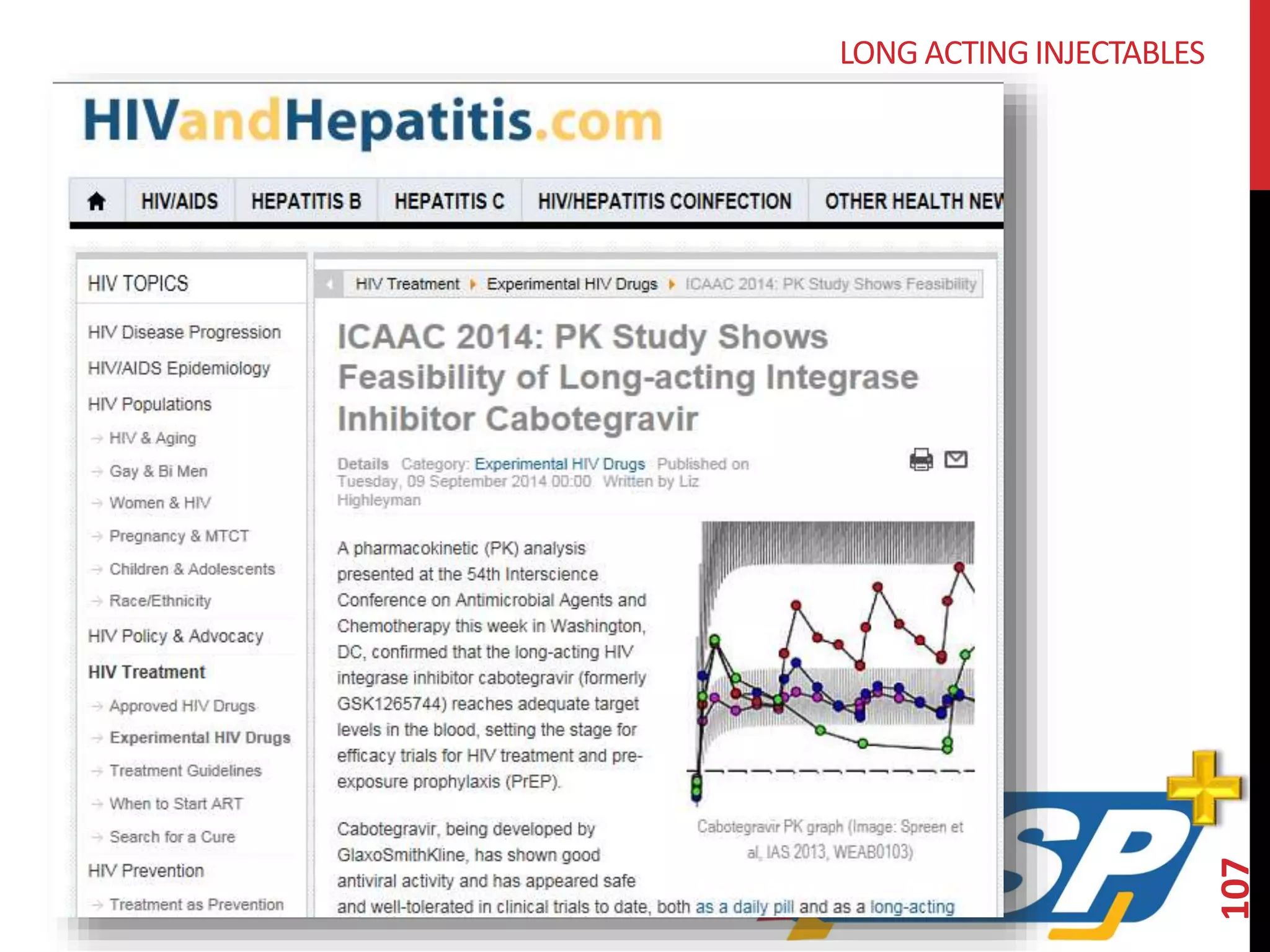
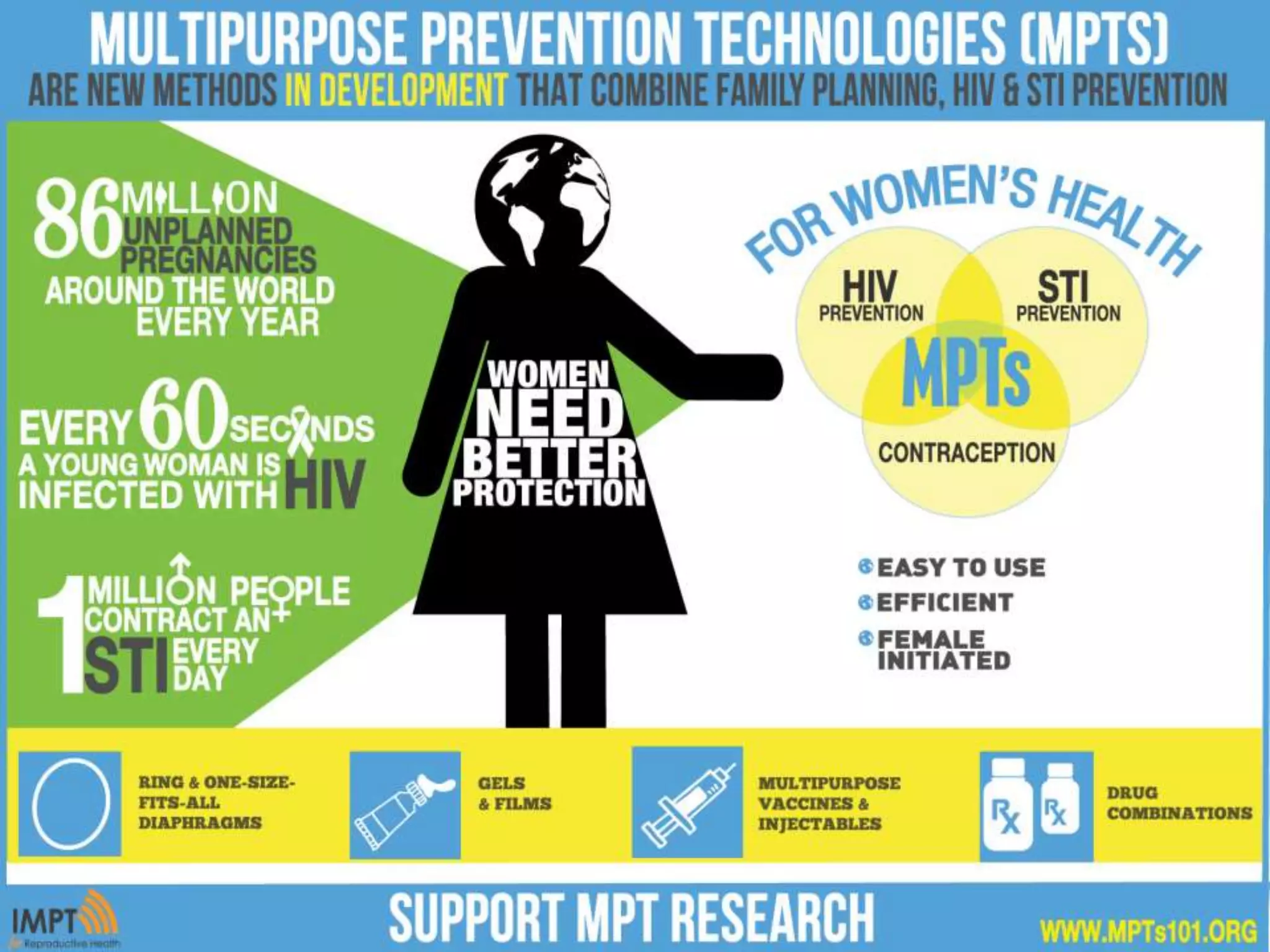
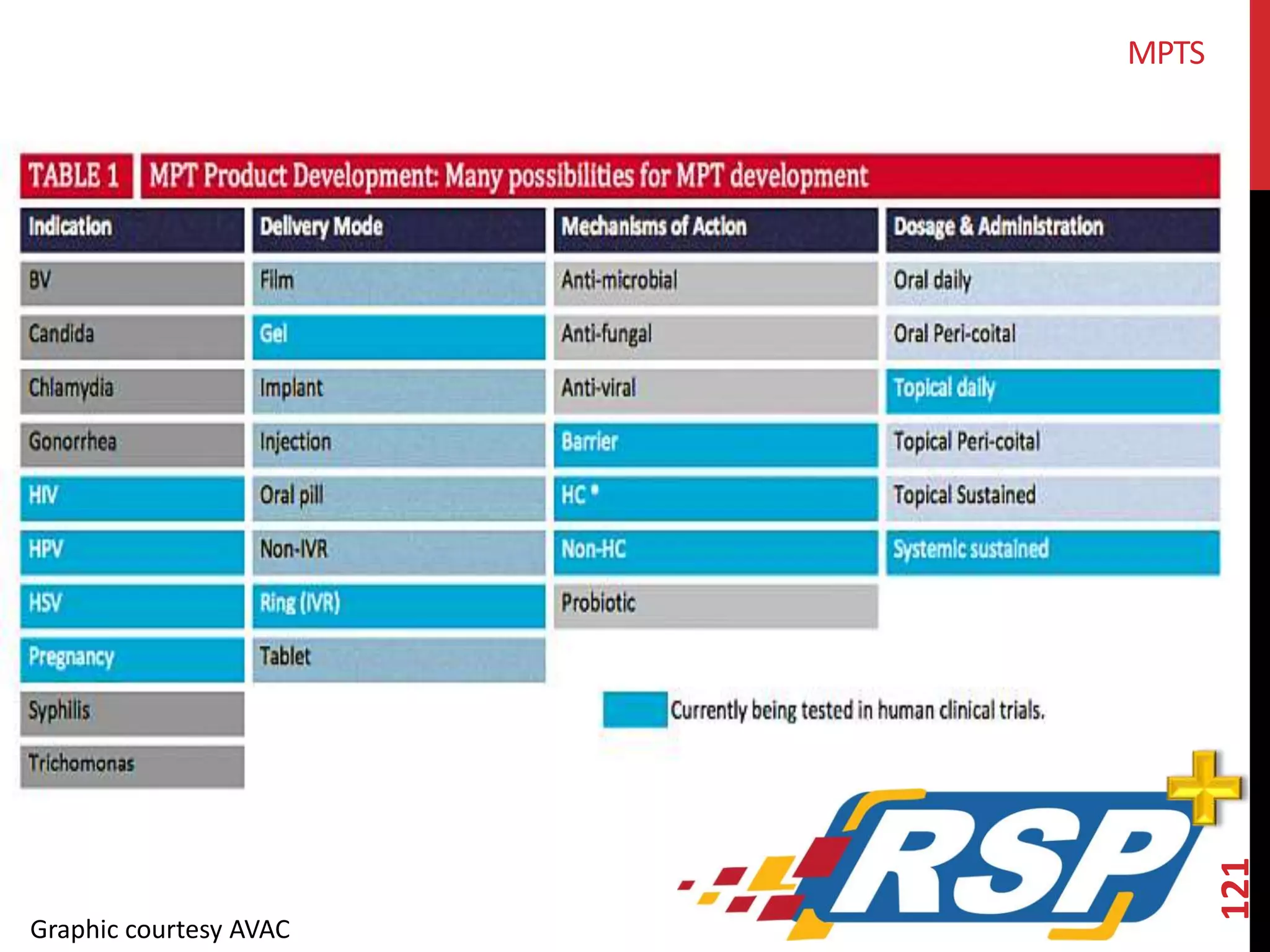
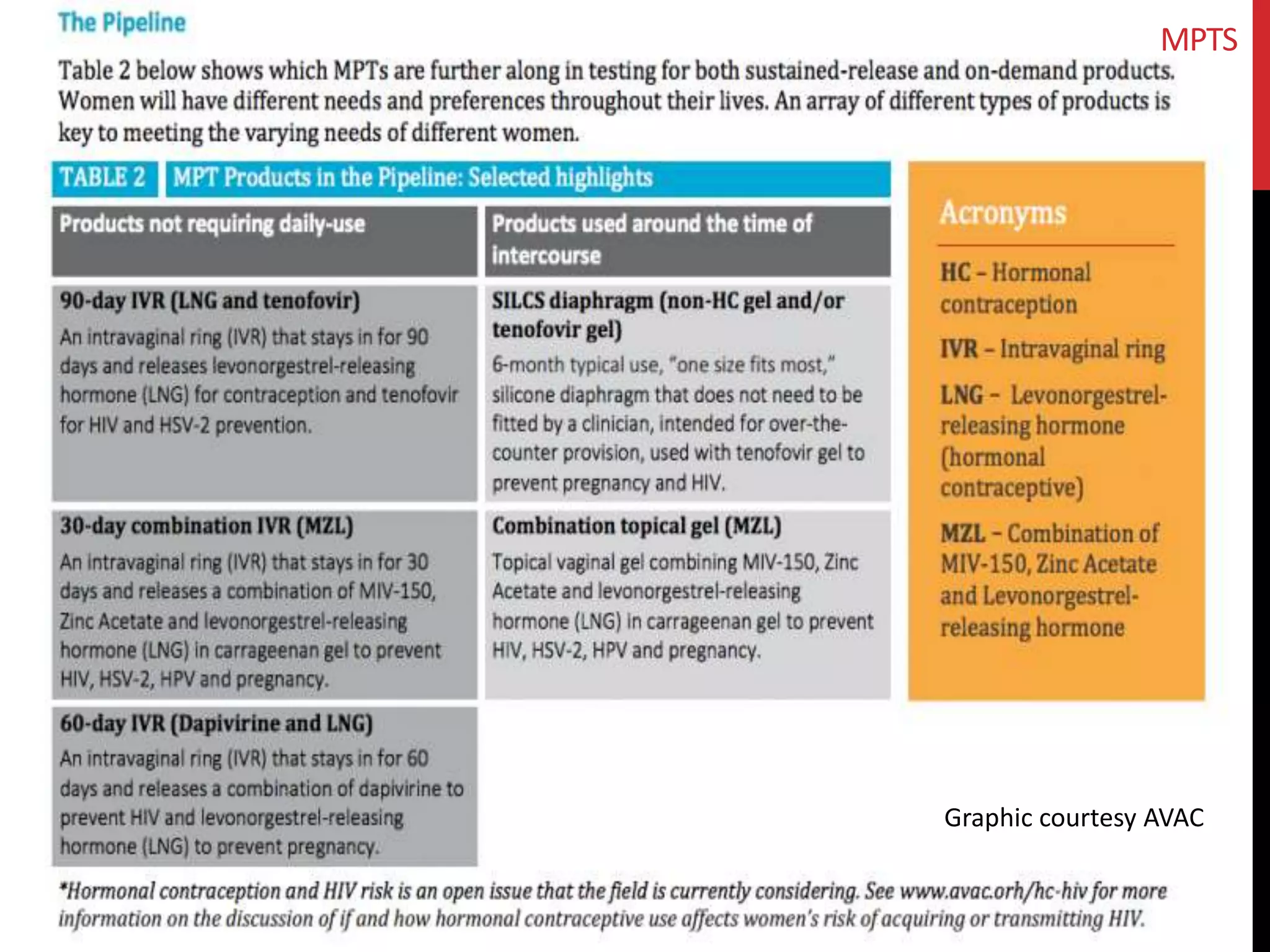
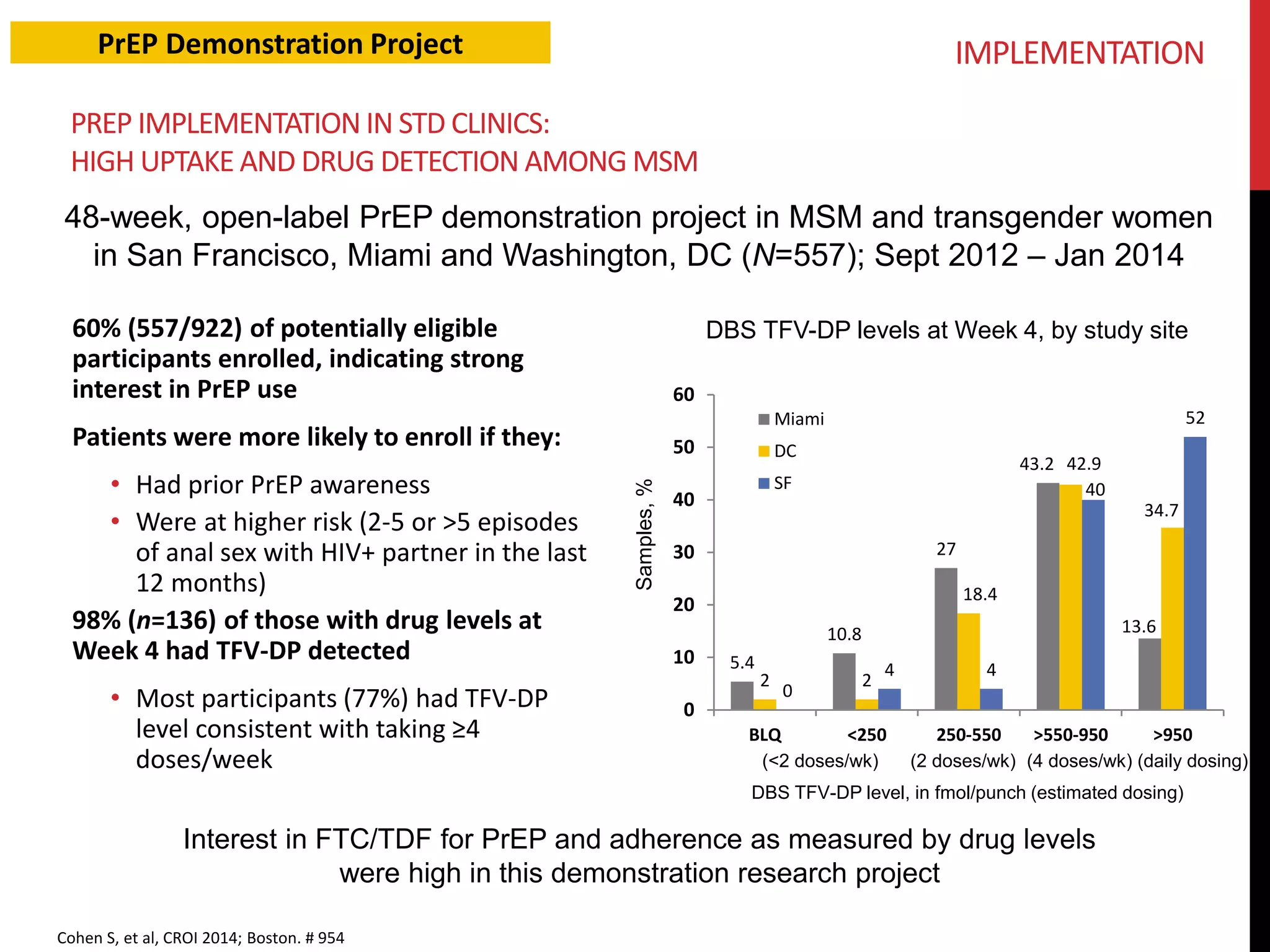
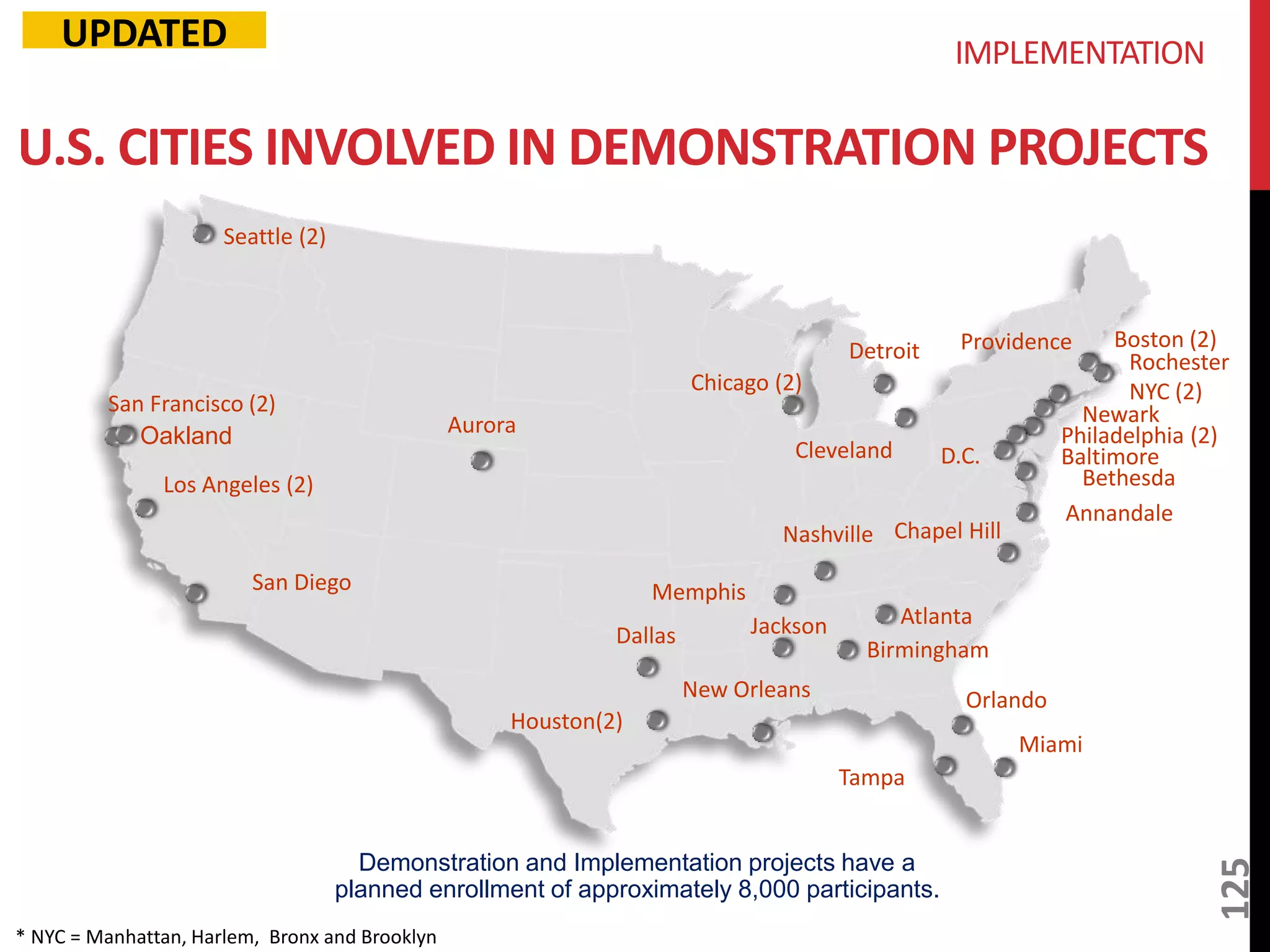
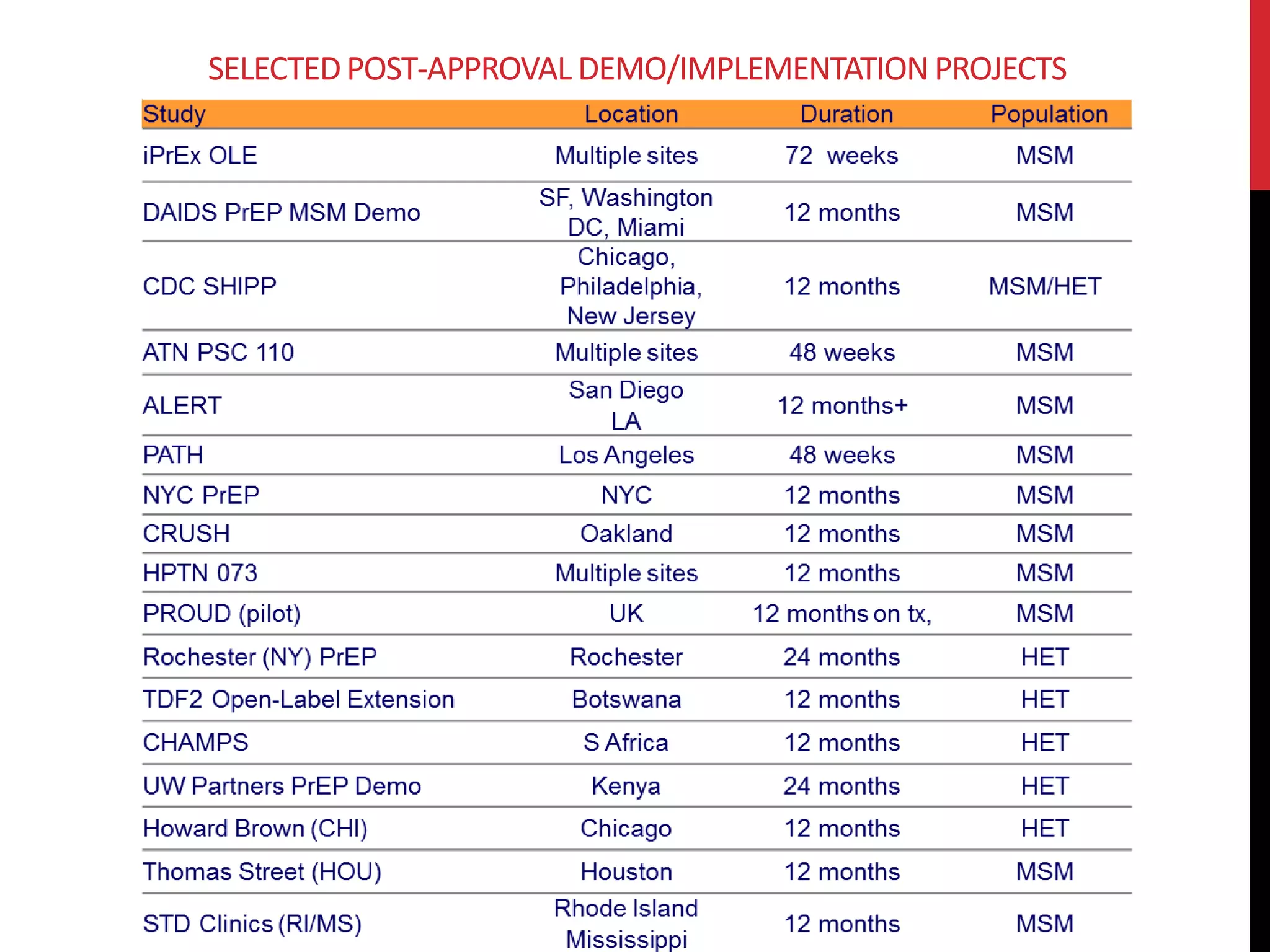
The document provides an overview of current advancements in HIV prevention technologies, including pre-exposure prophylaxis (PrEP) and rectal microbicides. It outlines various clinical trials, user demographics, insurance access issues, and ongoing research into different delivery methods and formulations for both vaginal and rectal applications. The agenda includes discussions on new dosing strategies, product implementations, and data on PrEP utilization among various populations.




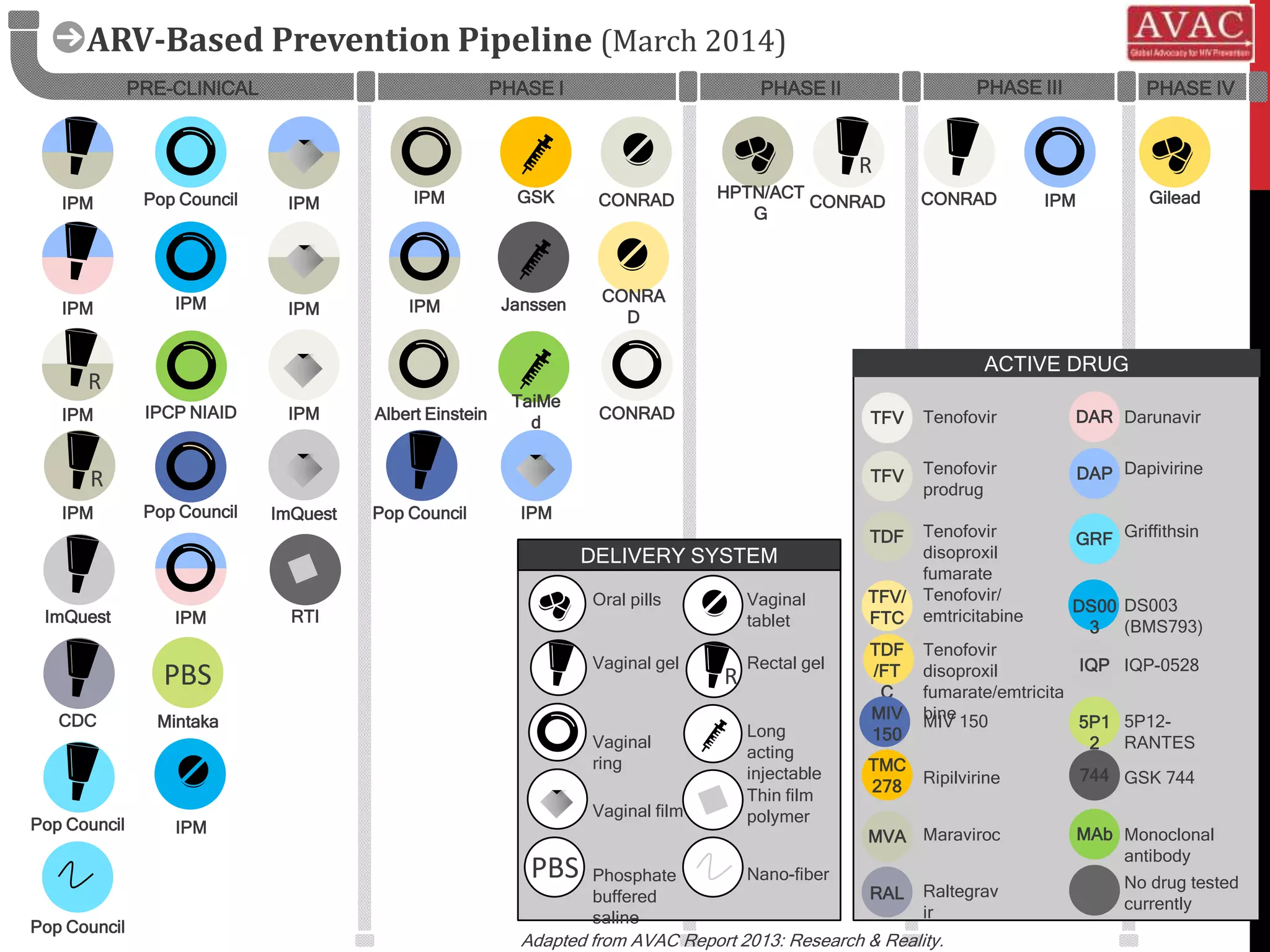












































![N = 192
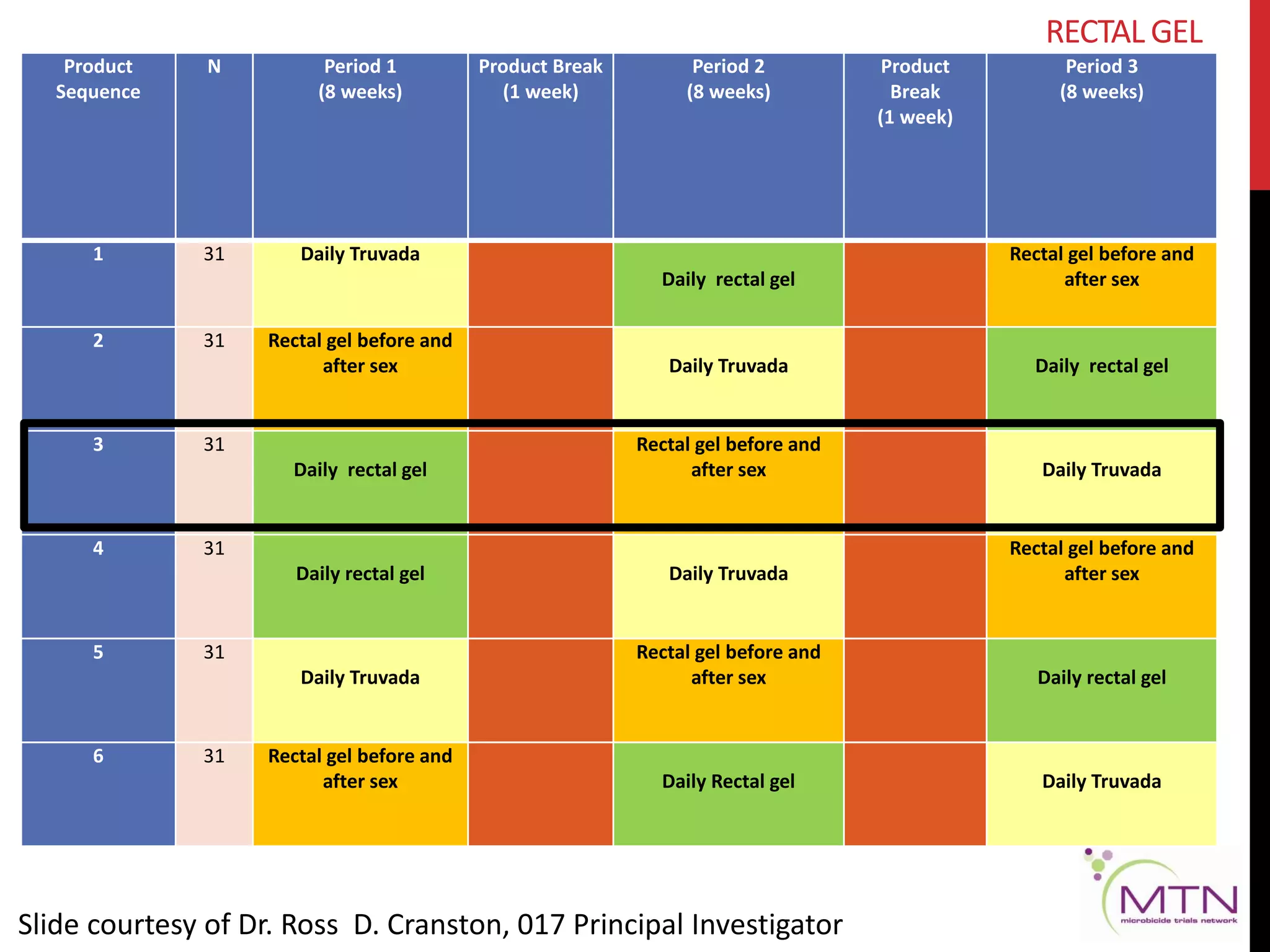
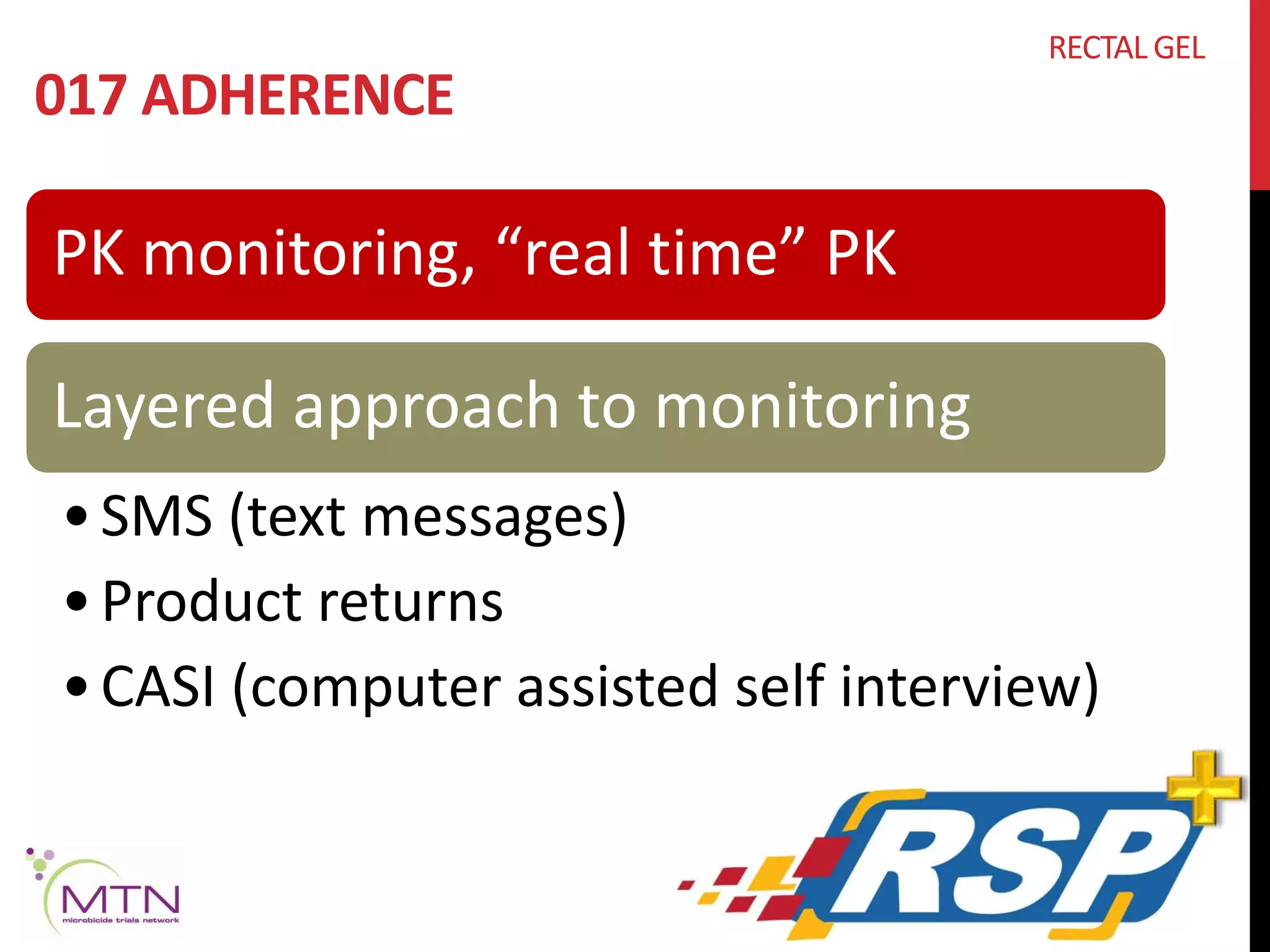
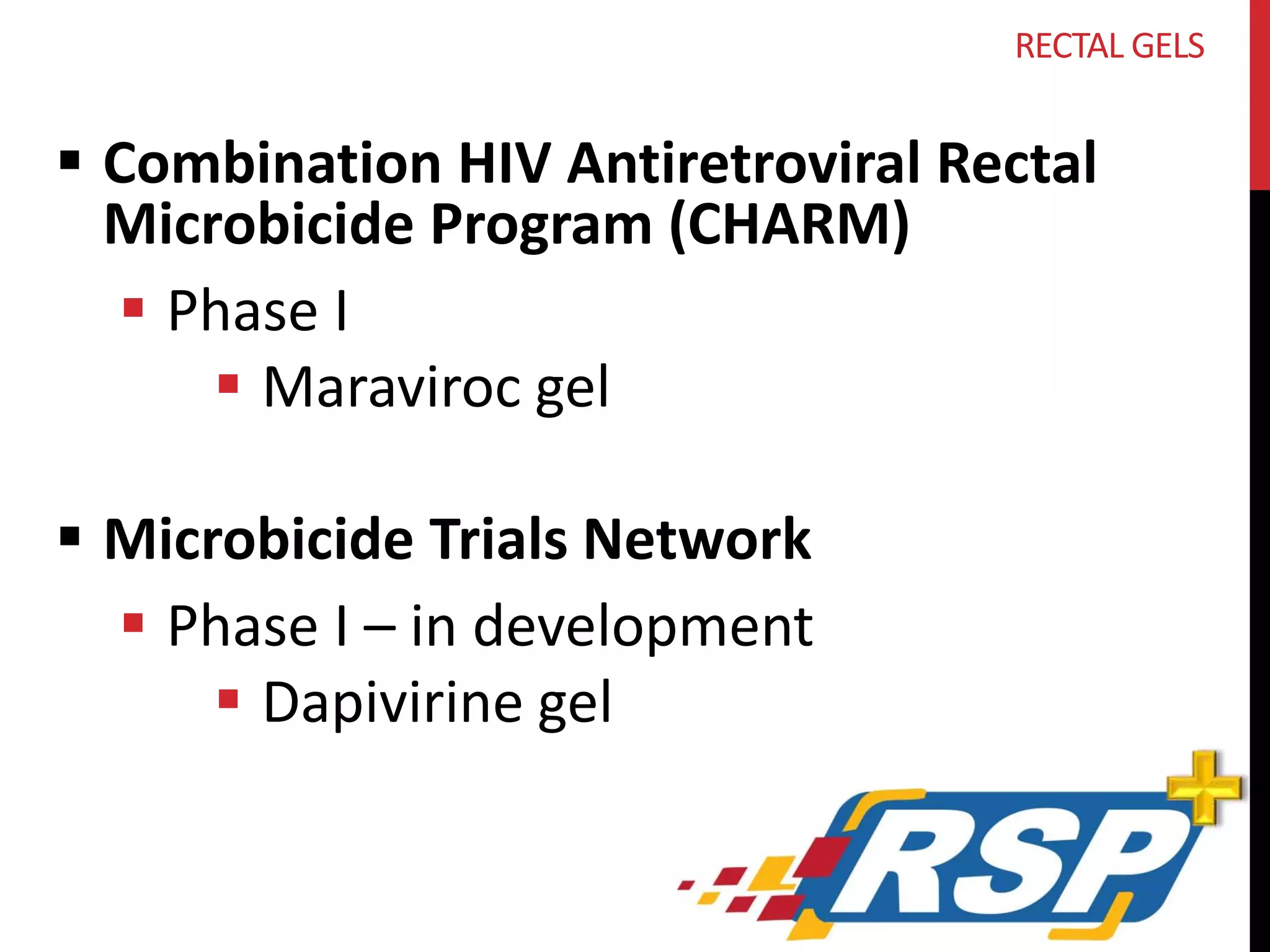
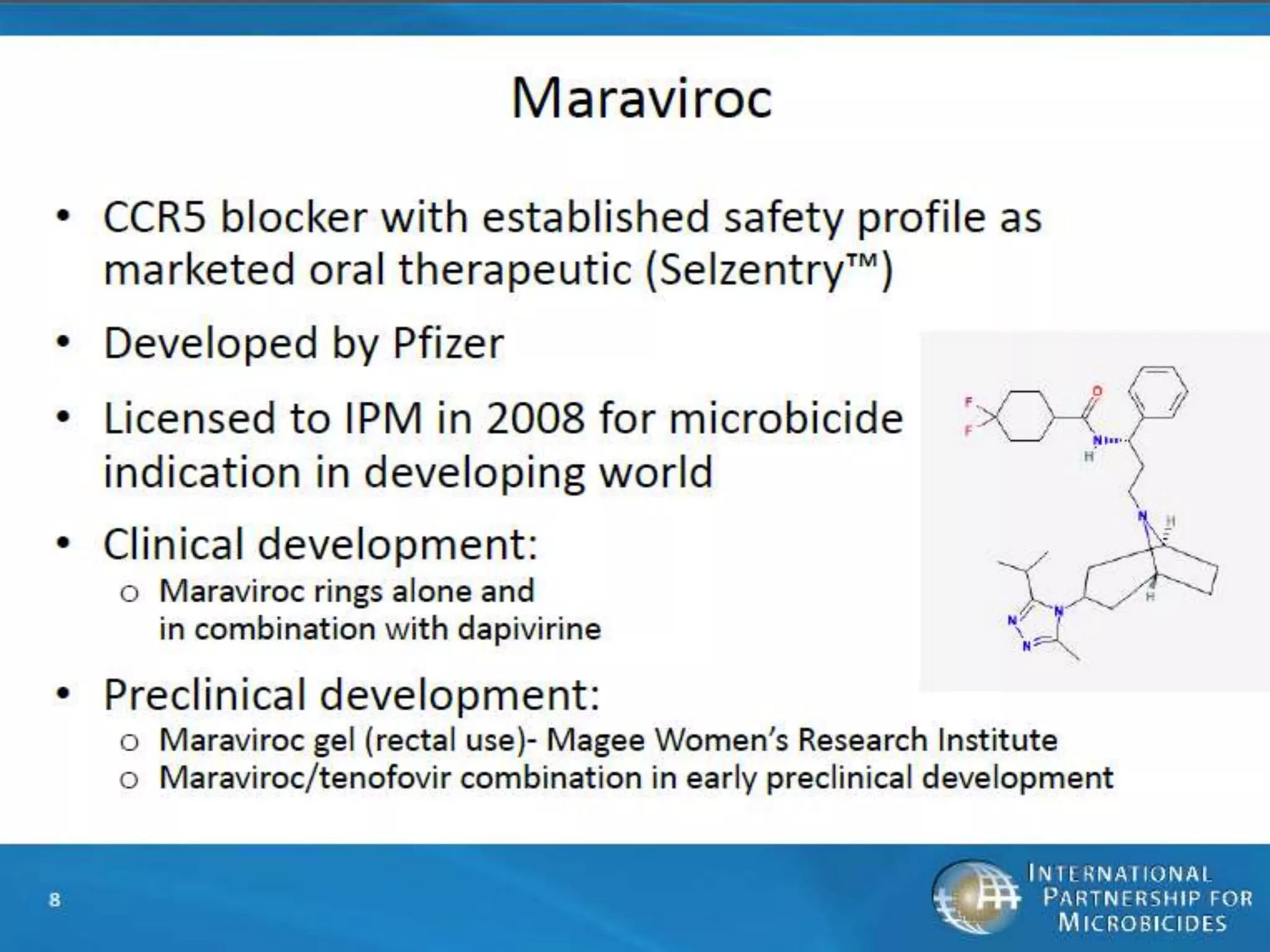
RECTAL GEL
[Sept ’13 – June ‘16 ]
Participants
Gay/MSM,
transgender
women
Study sites
• US (4)
• Thailand (2)
• RSA (1)
• Peru (1)](https://image.slidesharecdn.com/rsppipelinechicago10-141008161708-conversion-gate01/75/Exploring-the-Pipeline-Lubes-Rings-Films-Fibers-and-Shots-4-HIV-Prevention-64-2048.jpg)








![CAPRISA 008
Implementation study
underway
HIV-negative CAPRISA 004
participants
Effectiveness/sustainability
in rural and urban clinics
Oct ‘12 – Mar ’15
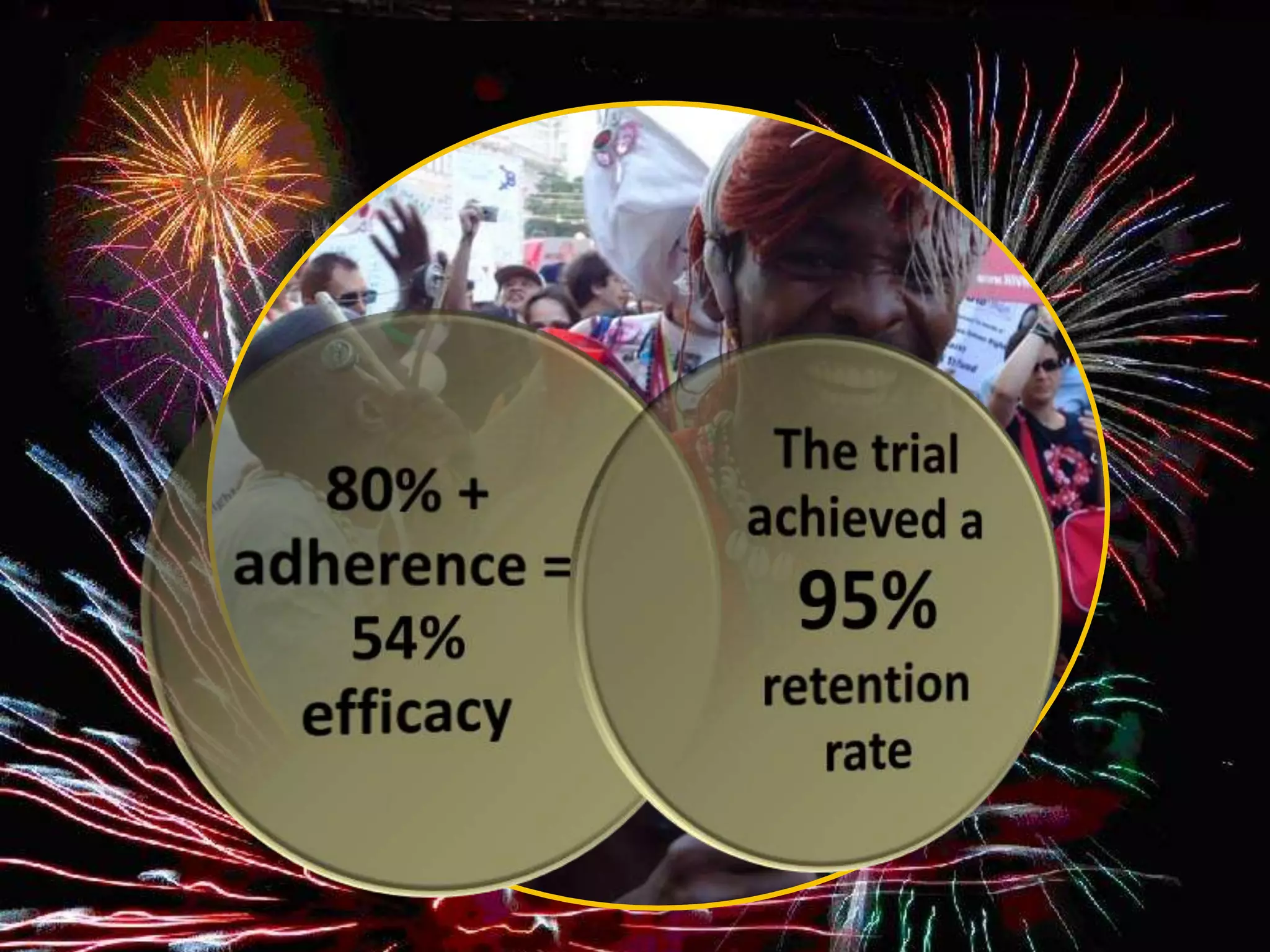
VOICE [MTN]
Phase 2B, randomized, double-blind,
placebo-controlled, 5-arm
trial
5029 women in trial – Uganda,
S. Africa, Zimbabwe
Studied daily use of following :
Vaginal tenofovir 1% gel
Oral tenofovir
Oral Truvada
Results announced March 2013
79
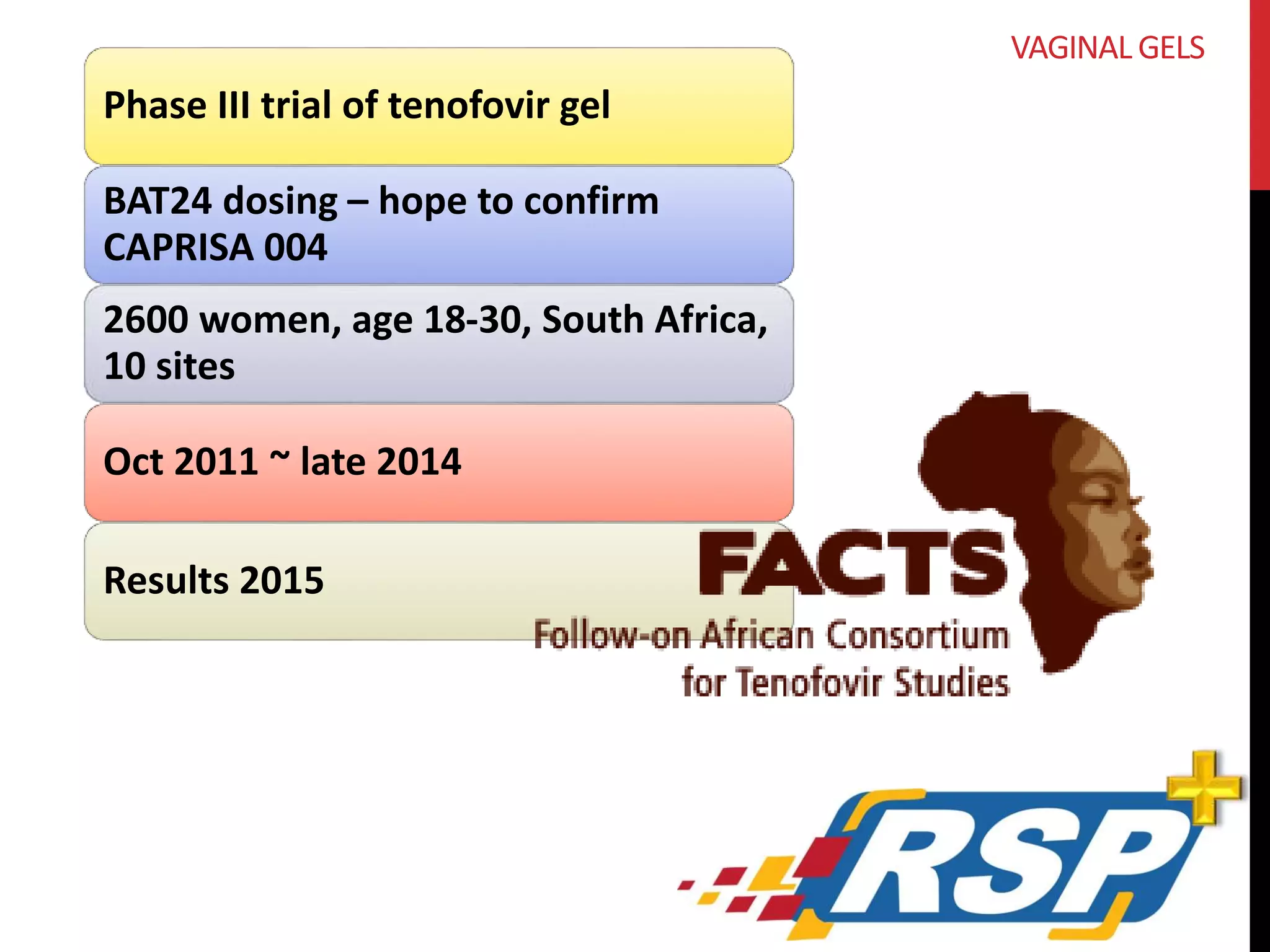
VAGINAL GELS](https://image.slidesharecdn.com/rsppipelinechicago10-141008161708-conversion-gate01/75/Exploring-the-Pipeline-Lubes-Rings-Films-Fibers-and-Shots-4-HIV-Prevention-79-2048.jpg)

































![129
SHIPP [SUSTAINABLE HEALTH CENTER
IMPLEMENTATION PREP PILOT]
June 1. 2014
Implementation project examining PrEP use in
primary care settings in Chicago, Newark, Houston,
and Philadelphia
Serves women and men
Access Grand Boulevard Specialty Clinic
5401 South Wentworth Avenue
PS-PREP
Jan 1, 2015
Randomized clinical trial of
PrEP linkage program by DIS
staff, UC and CDPH
CHICAG0 IMPLEMENTATION](https://image.slidesharecdn.com/rsppipelinechicago10-141008161708-conversion-gate01/75/Exploring-the-Pipeline-Lubes-Rings-Films-Fibers-and-Shots-4-HIV-Prevention-129-2048.jpg)





![What is most striking is
that [the] future is not
driven by the science, it
is driven by communities
and the needs of
implementation.](https://image.slidesharecdn.com/rsppipelinechicago10-141008161708-conversion-gate01/75/Exploring-the-Pipeline-Lubes-Rings-Films-Fibers-and-Shots-4-HIV-Prevention-135-2048.jpg)