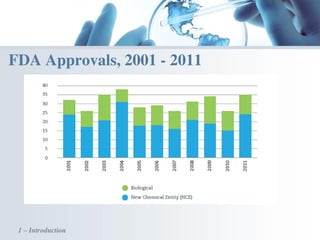
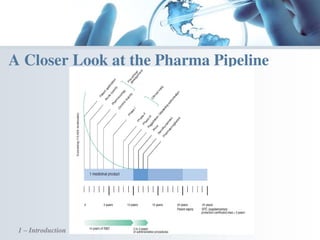
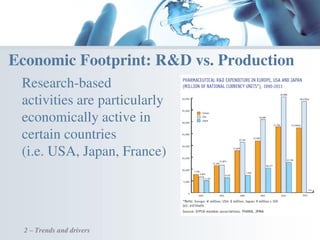
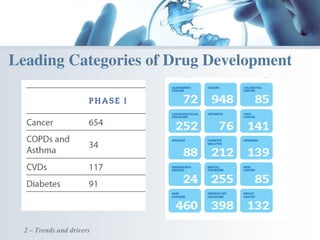
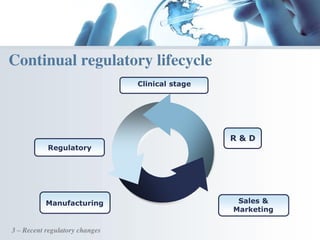
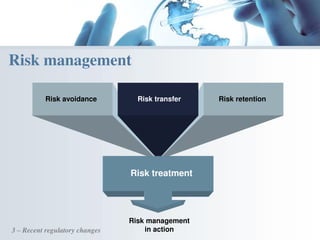
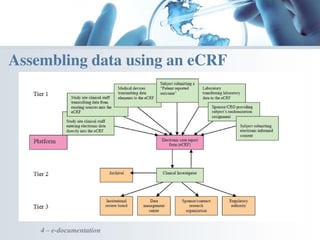
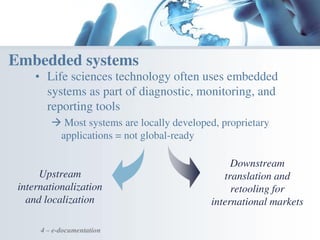
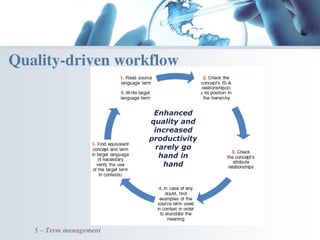
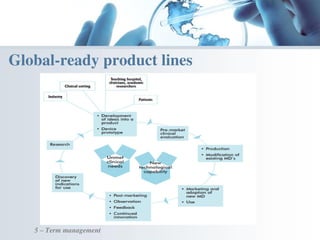
The document discusses the evolving landscape of medical translation, driven by technological advancements and regulatory changes, highlighting the need for translators to adapt to tools that enhance accuracy and efficiency in data processing. It emphasizes the shift from traditional paper-based systems to electronic documentation and case report forms in clinical trials, which necessitates standardized data input and harmonization across global teams. As the pharmaceutical industry continues to expand into 'pharmerging' markets, medical translators must embrace these innovations to remain competitive and ensure compliance with evolving standards.