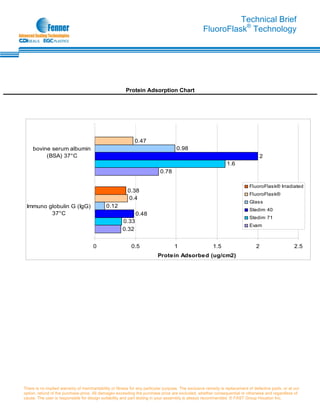
FluoroFlask® technology provides a high-purity, robust fluid packaging solution using an economic, high-performance fluoropolymer material as an alternative to conventional multilayer bladder products. FluoroFlask® products are produced through a proprietary process that manipulates fluoropolymers into thin-walled containers with various shapes and sizes. This allows elimination of post-mold welding and improves efficiency over conventional multilayer products. FluoroFlask® products offer advantages for applications in semiconductors, chemicals, biopharmaceuticals, and drugs due to their chemical inertness, low permeability, high purity, and ability to withstand temperature cycling.