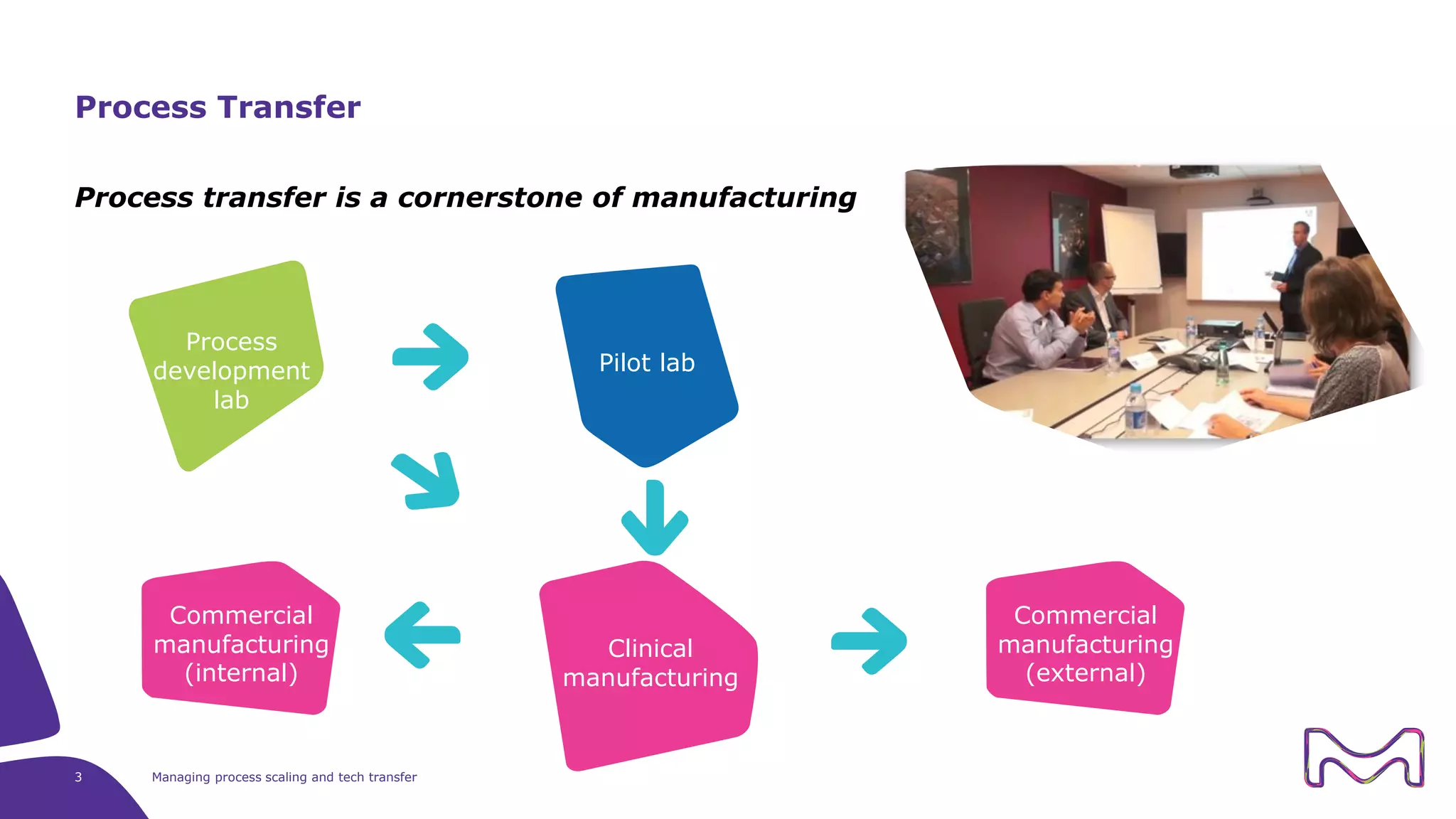
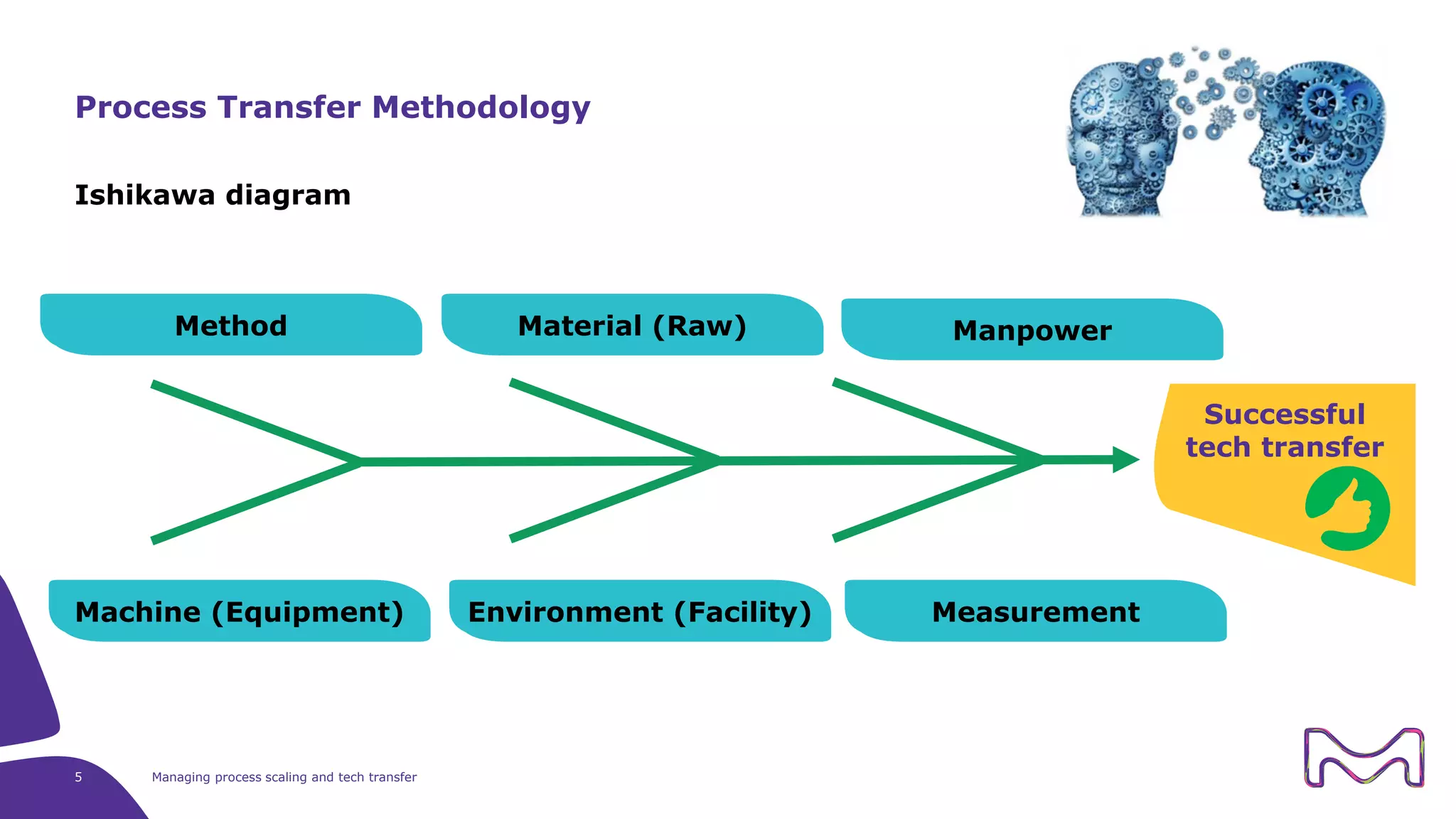
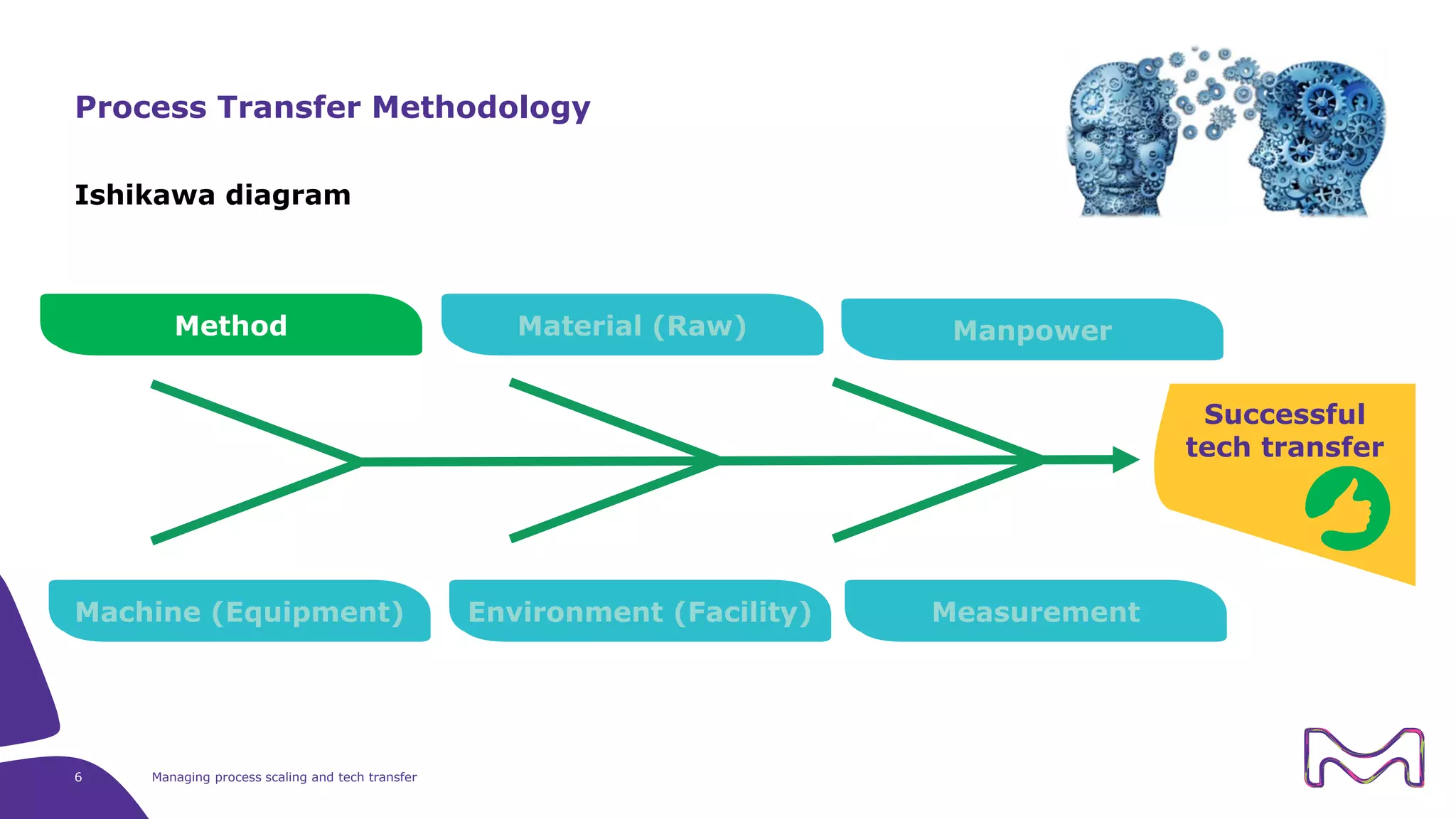
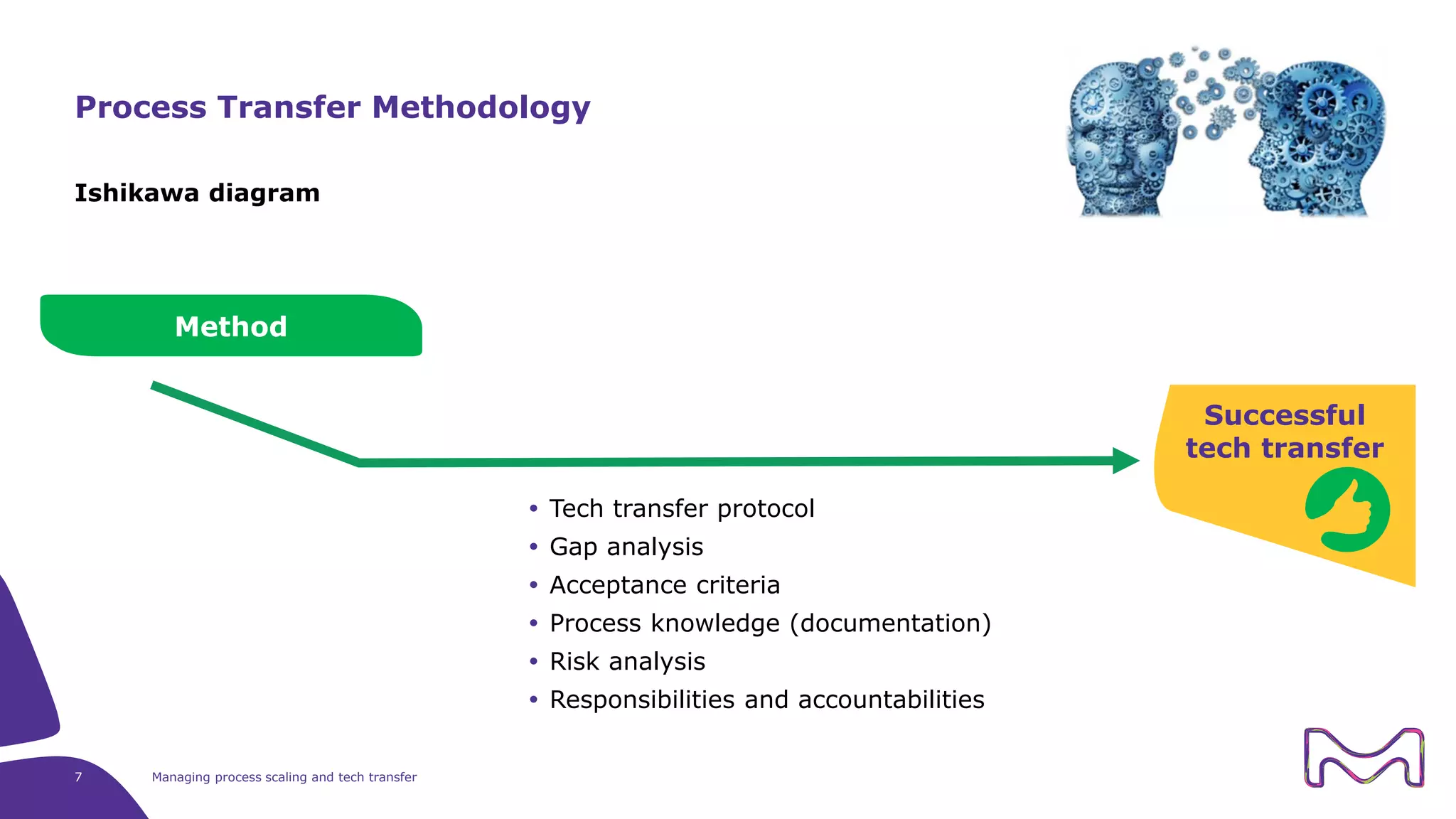
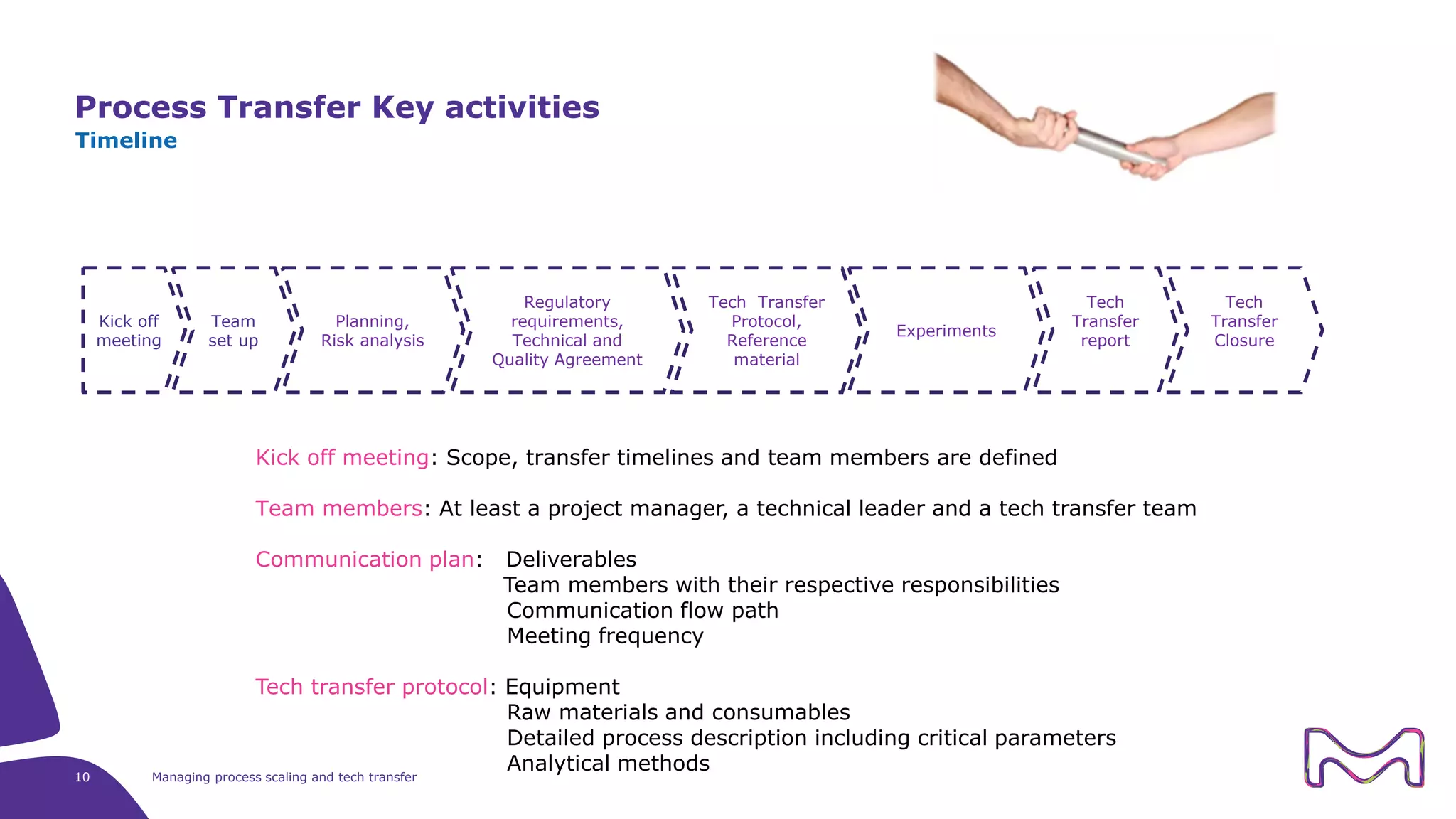
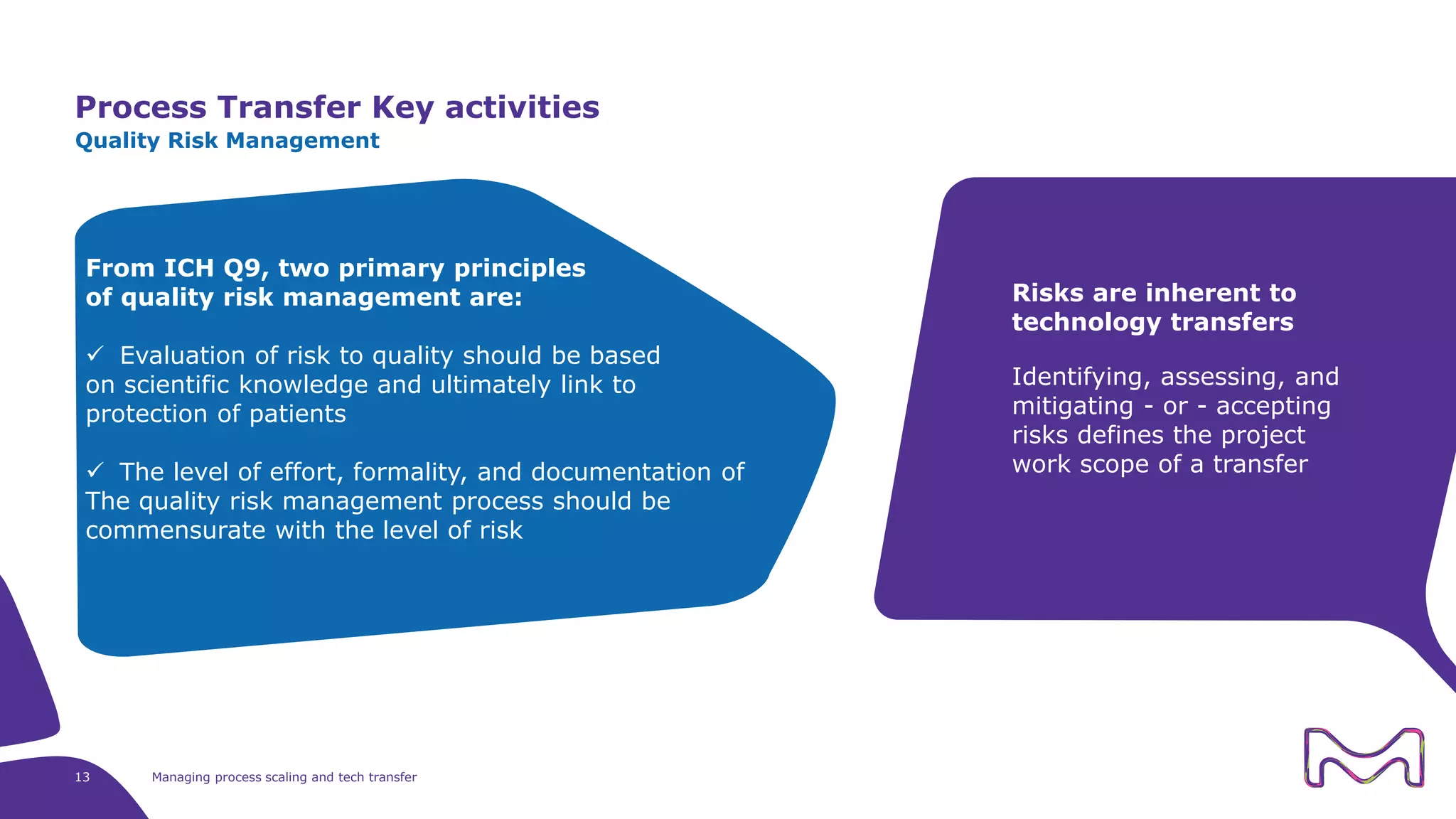
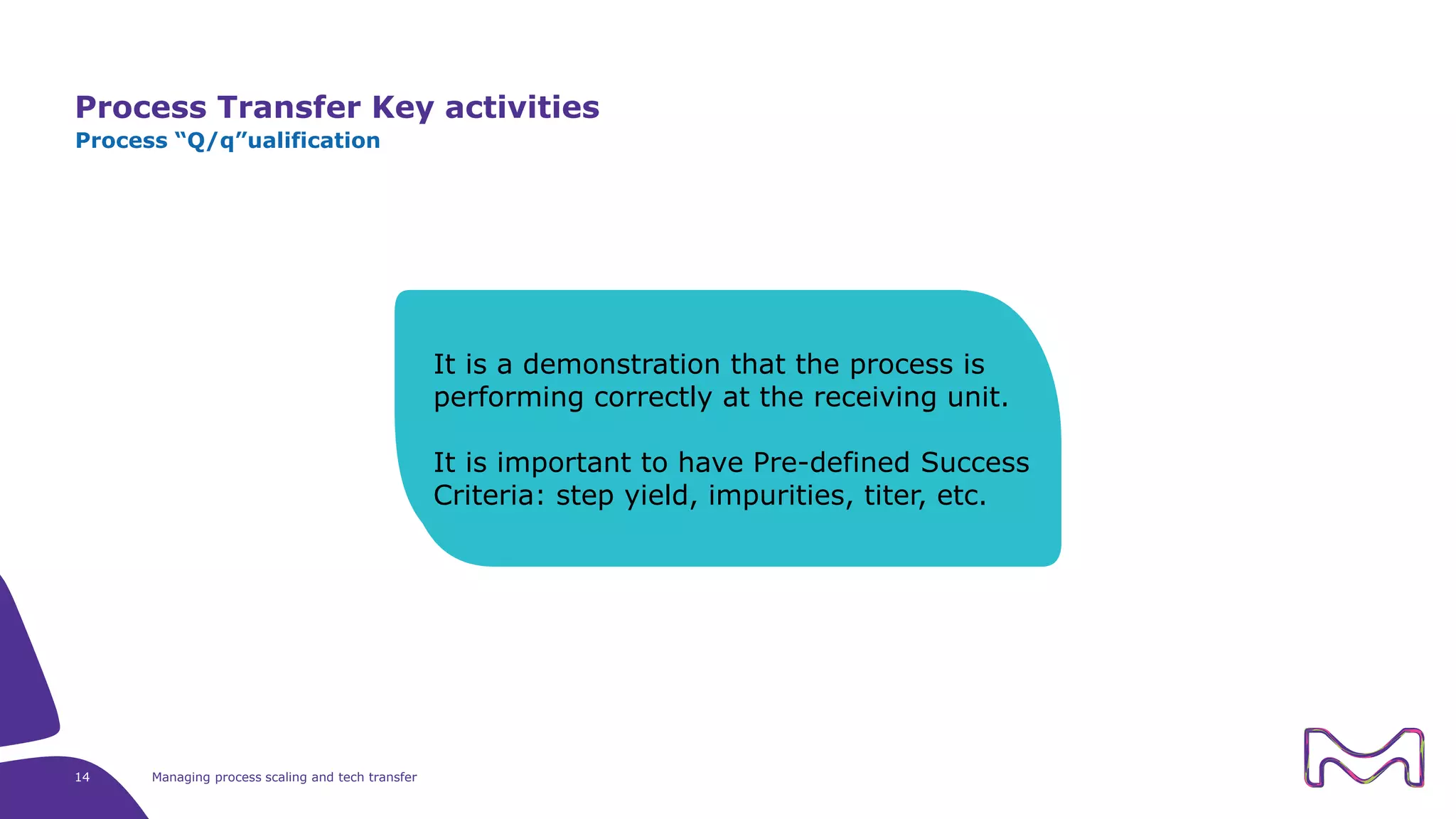
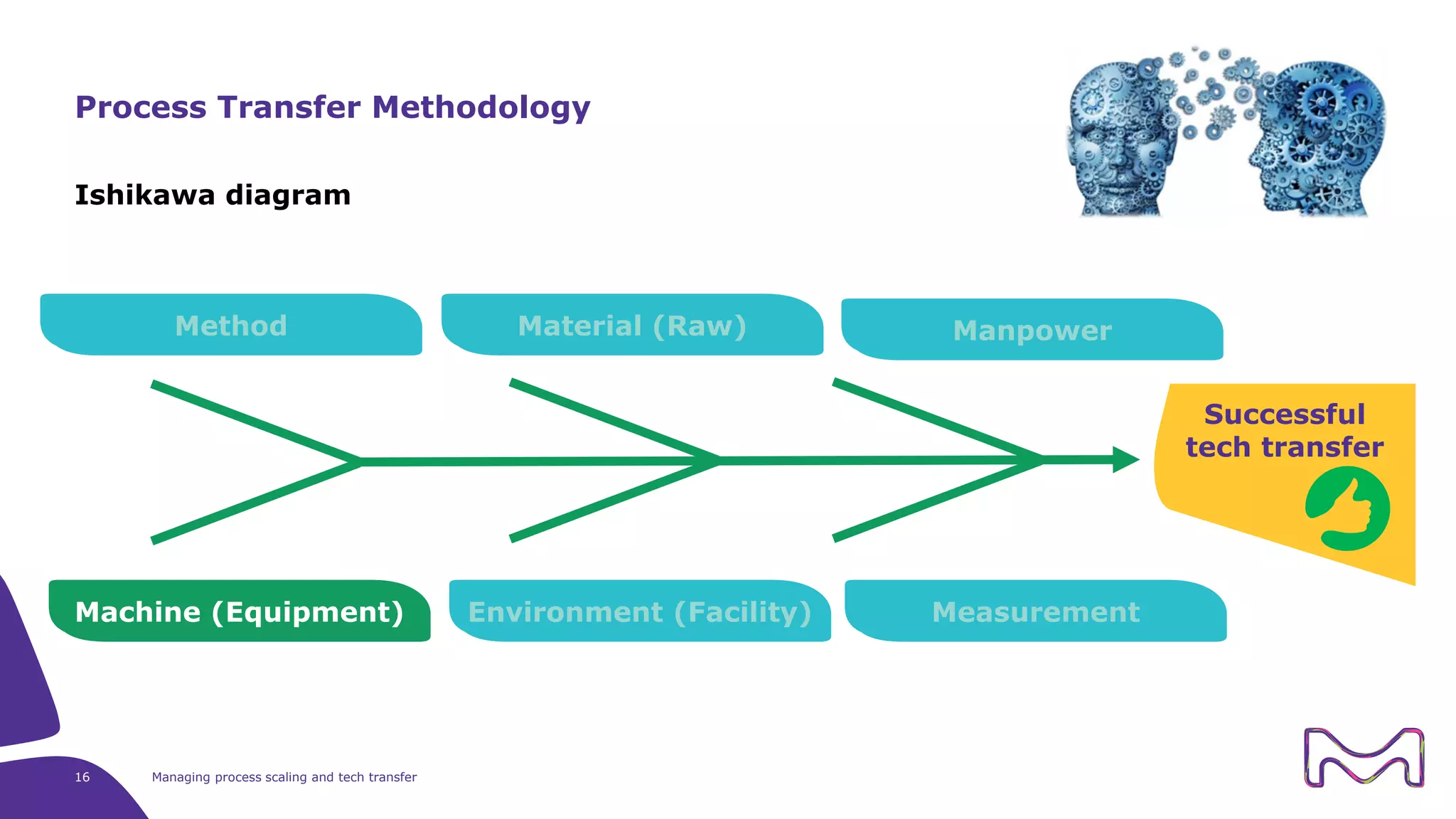
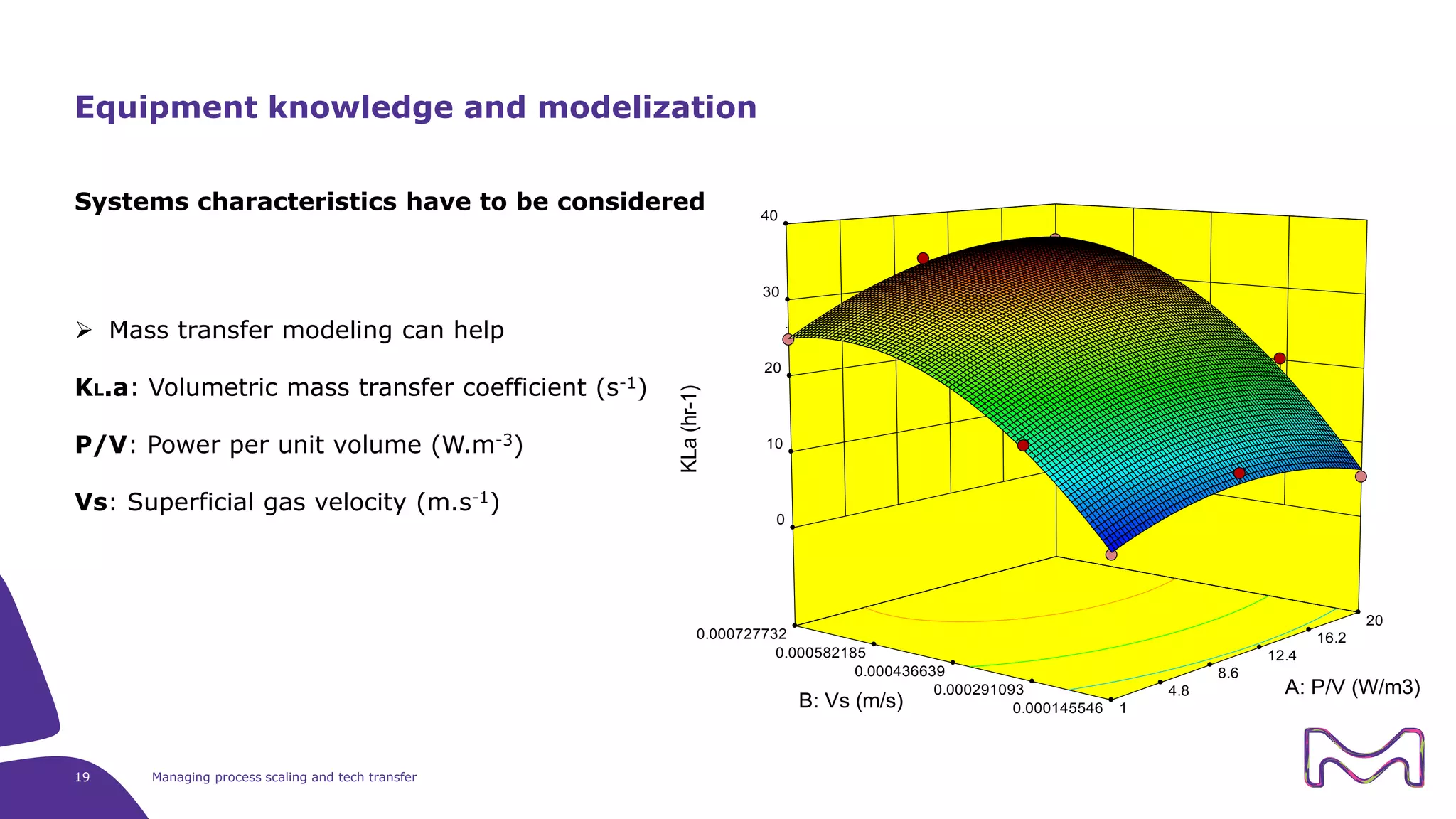
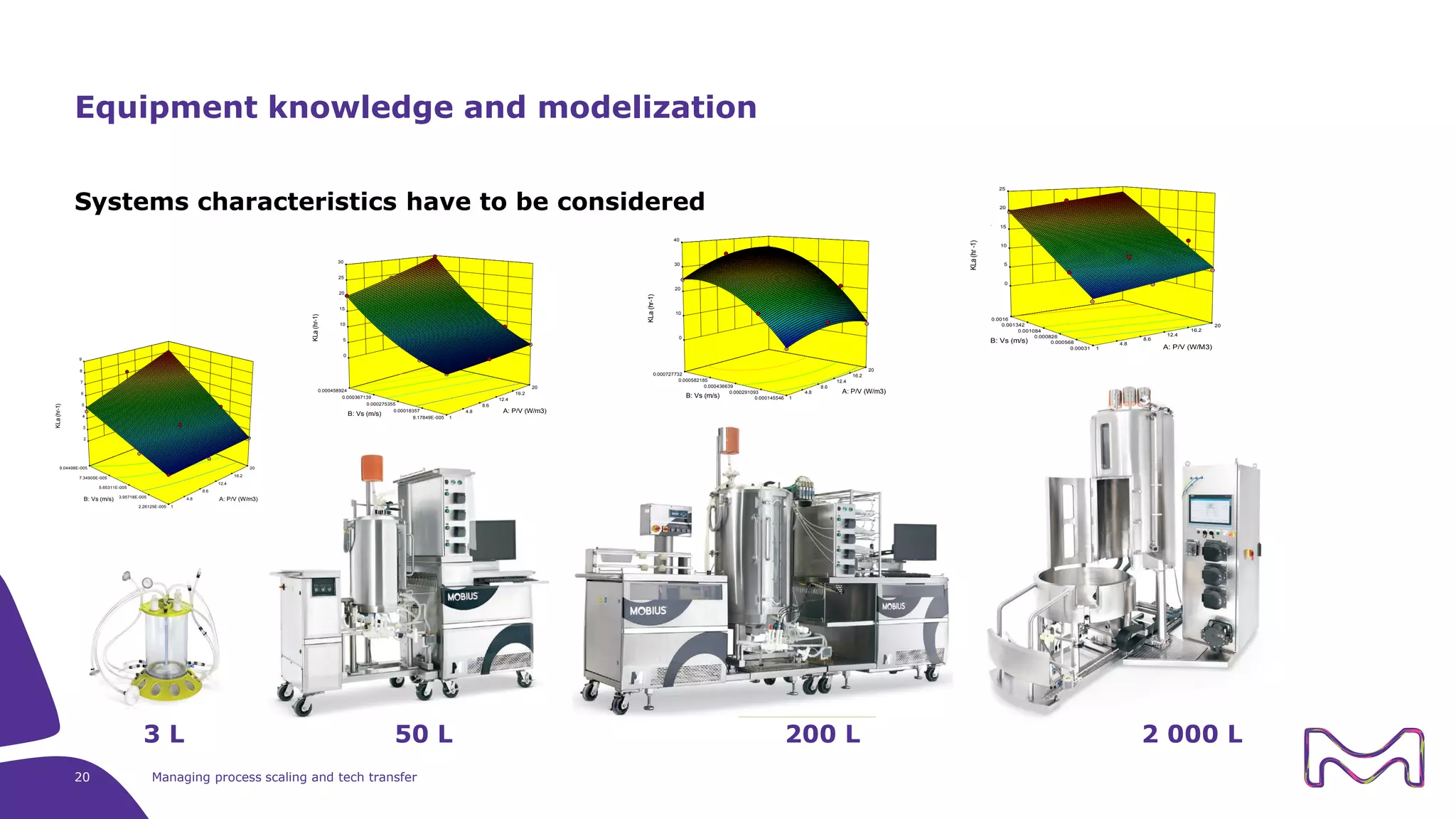
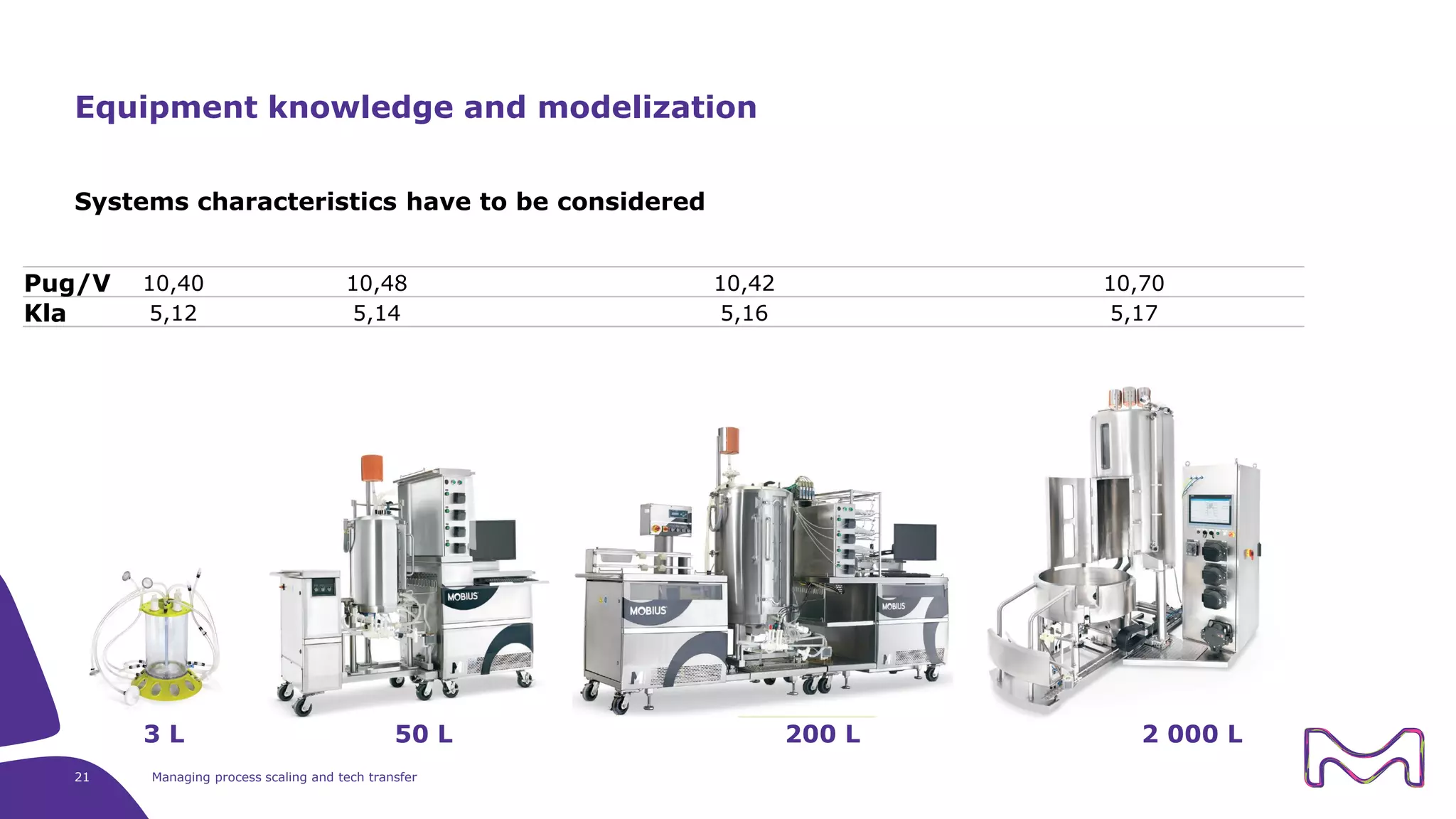
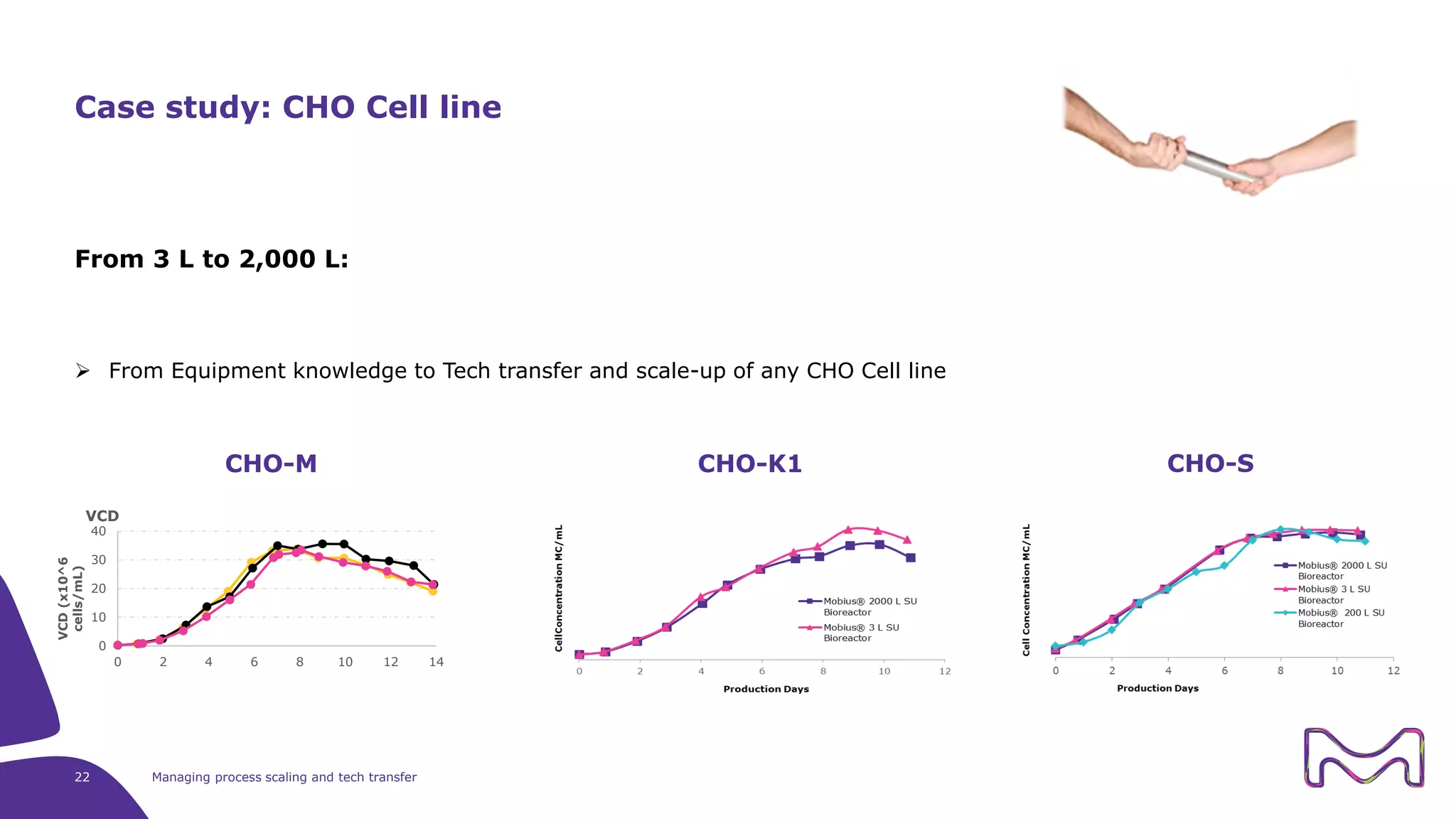
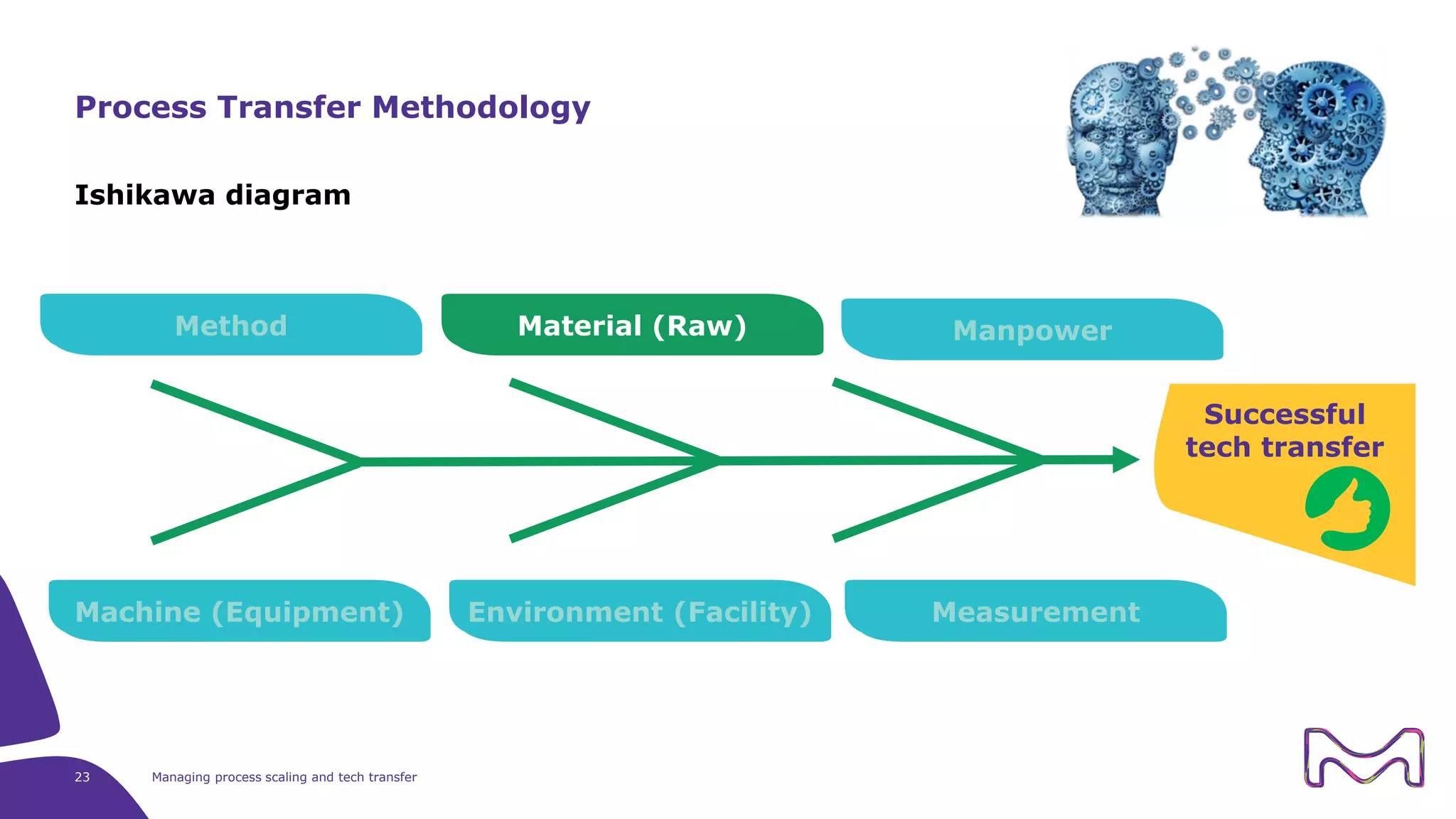
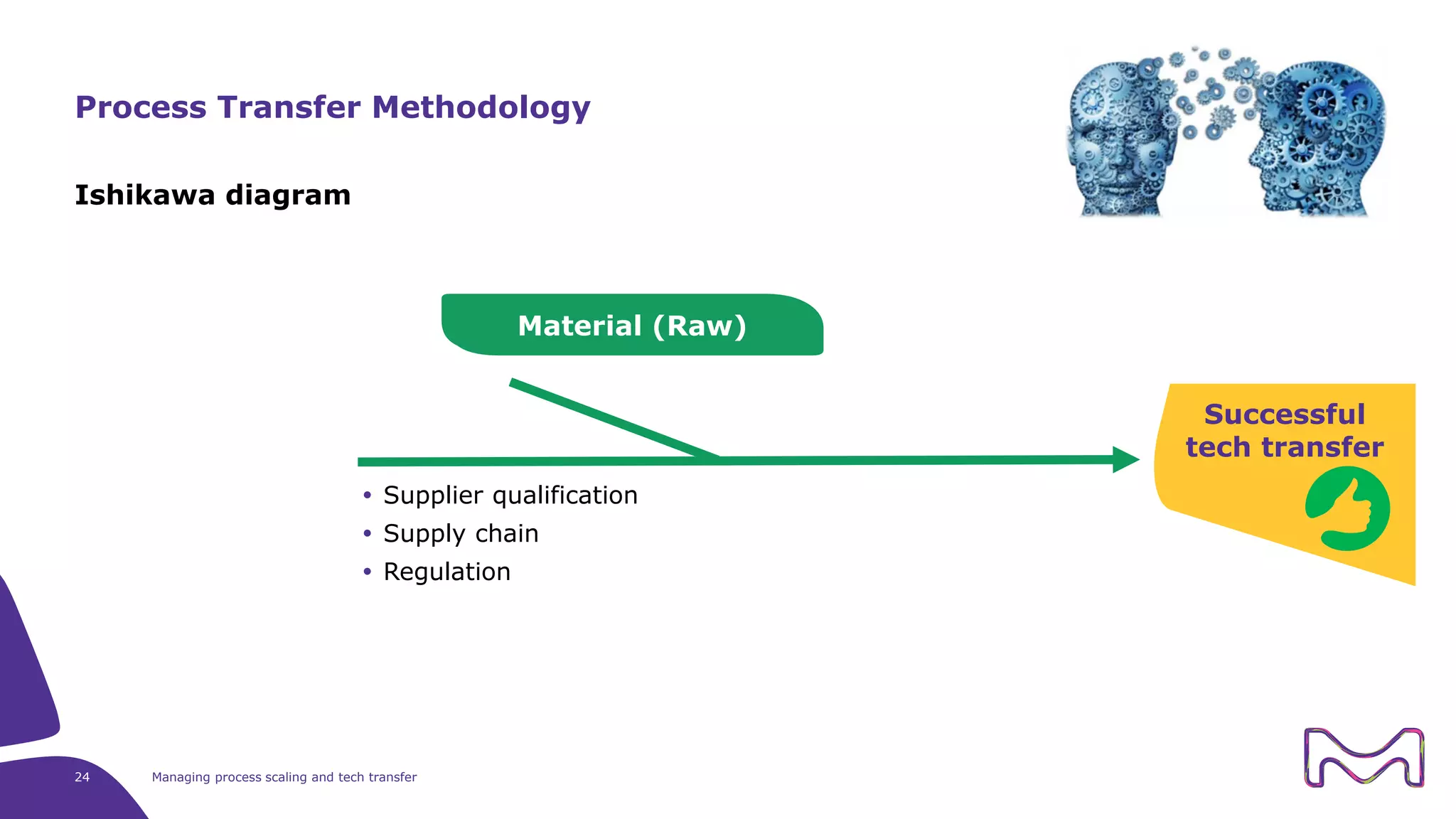
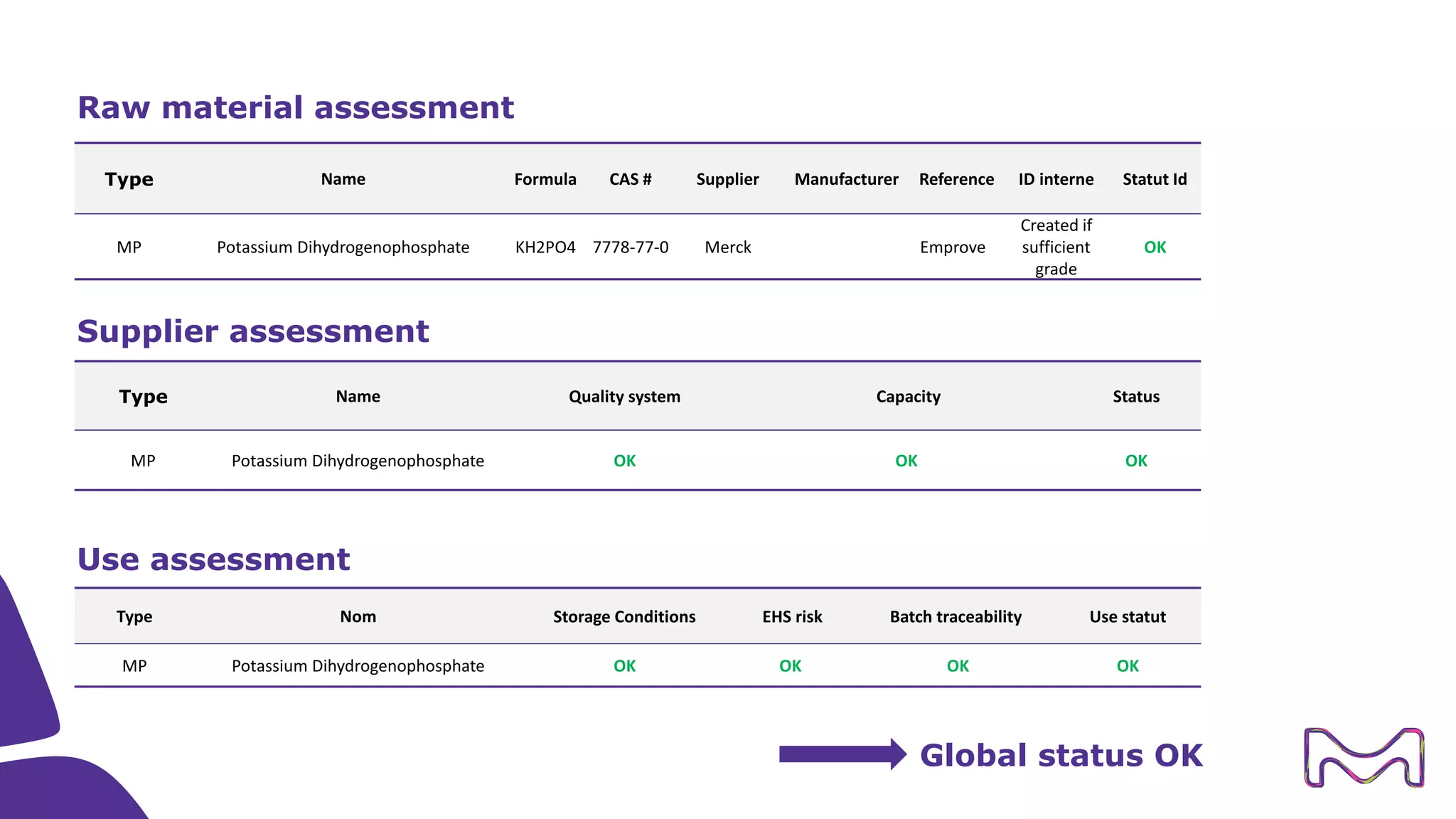
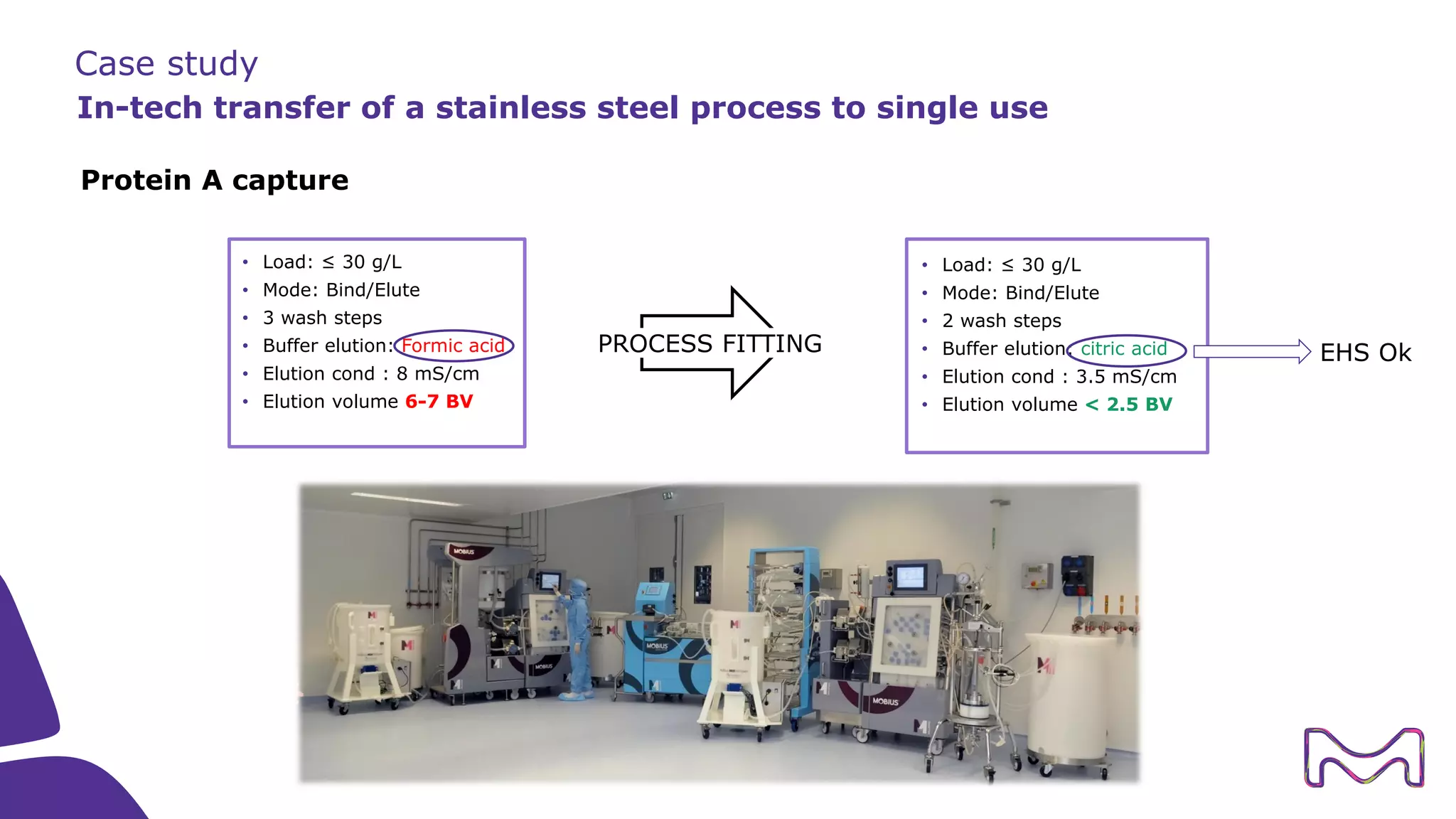
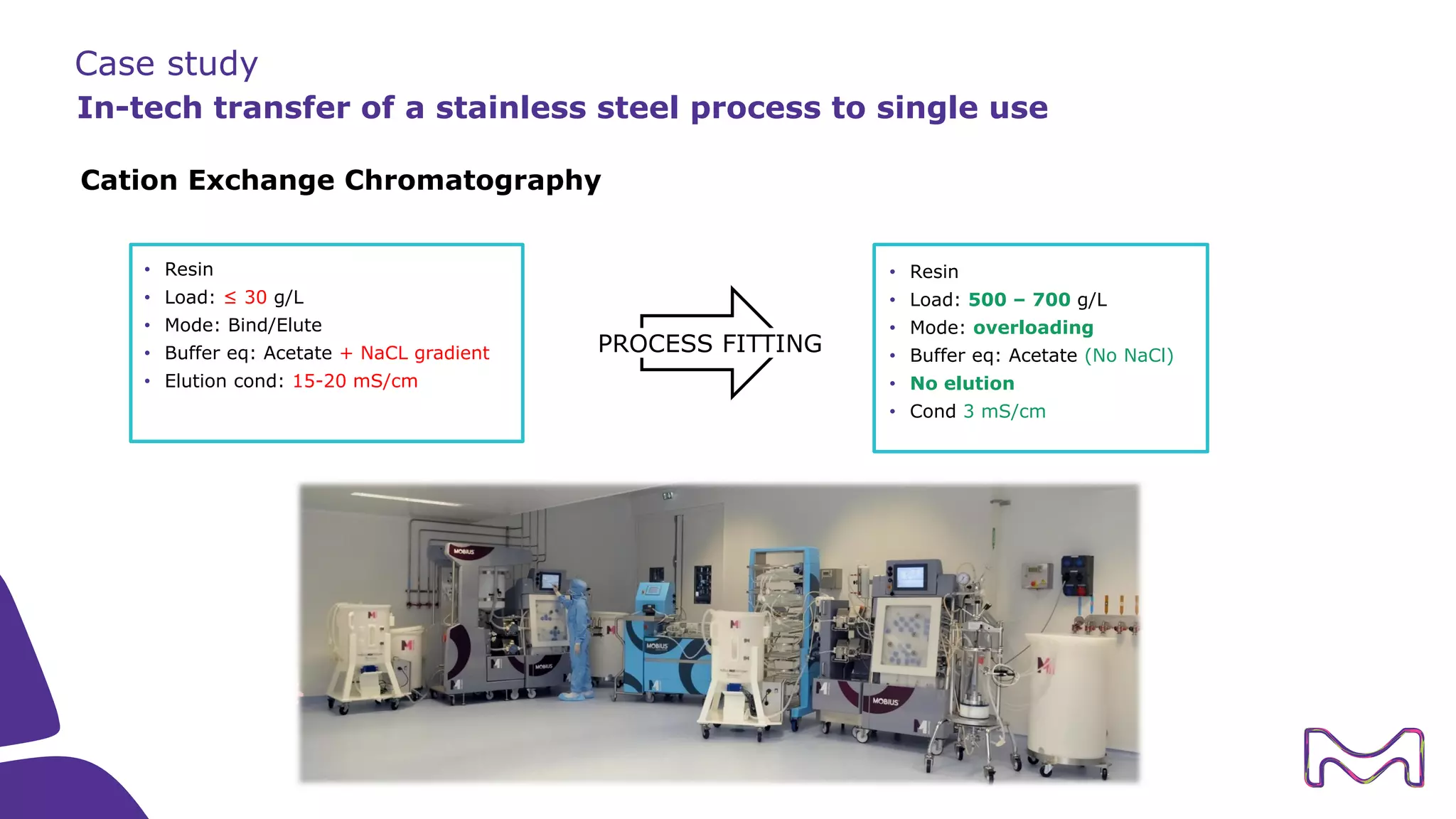
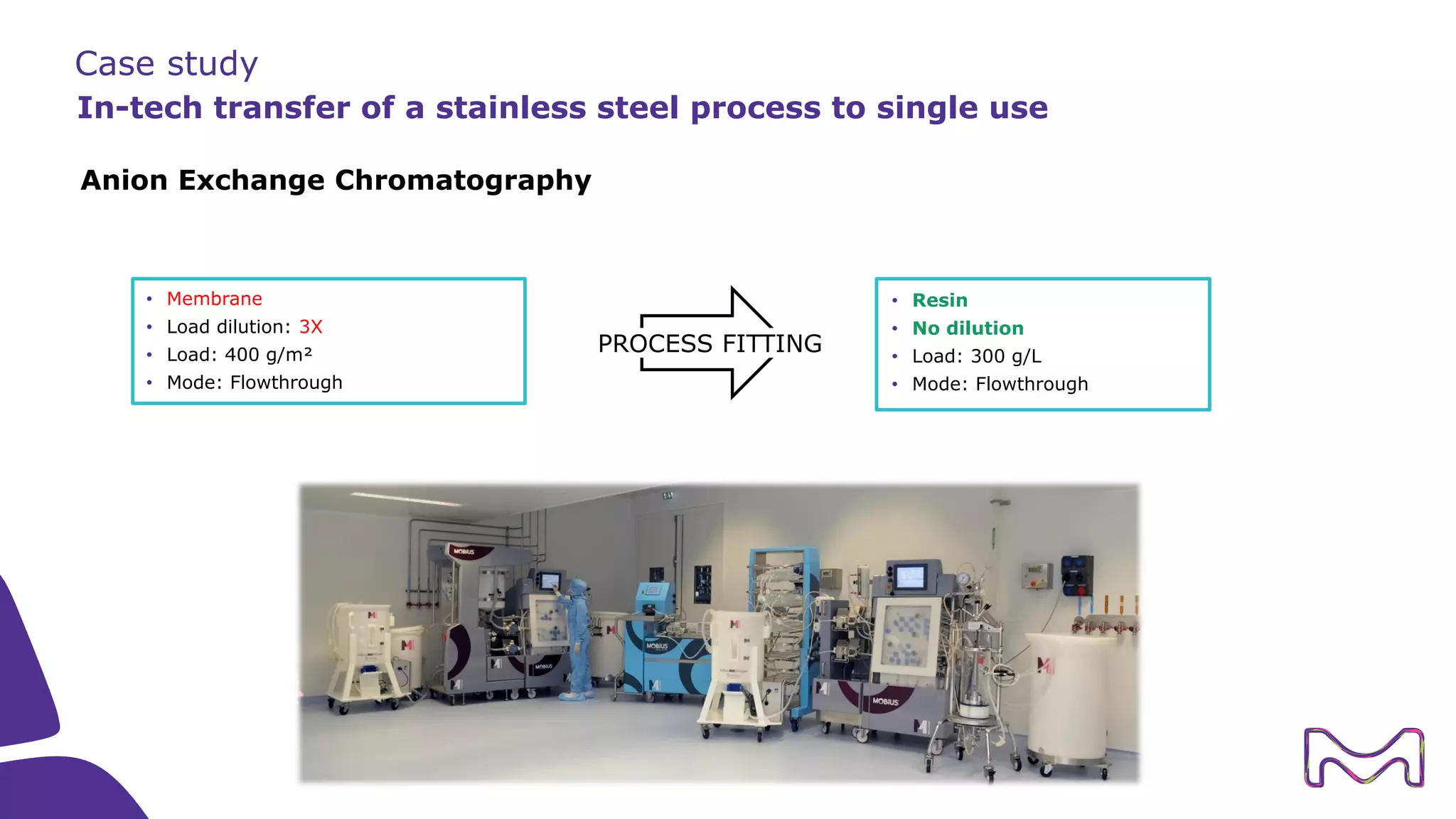
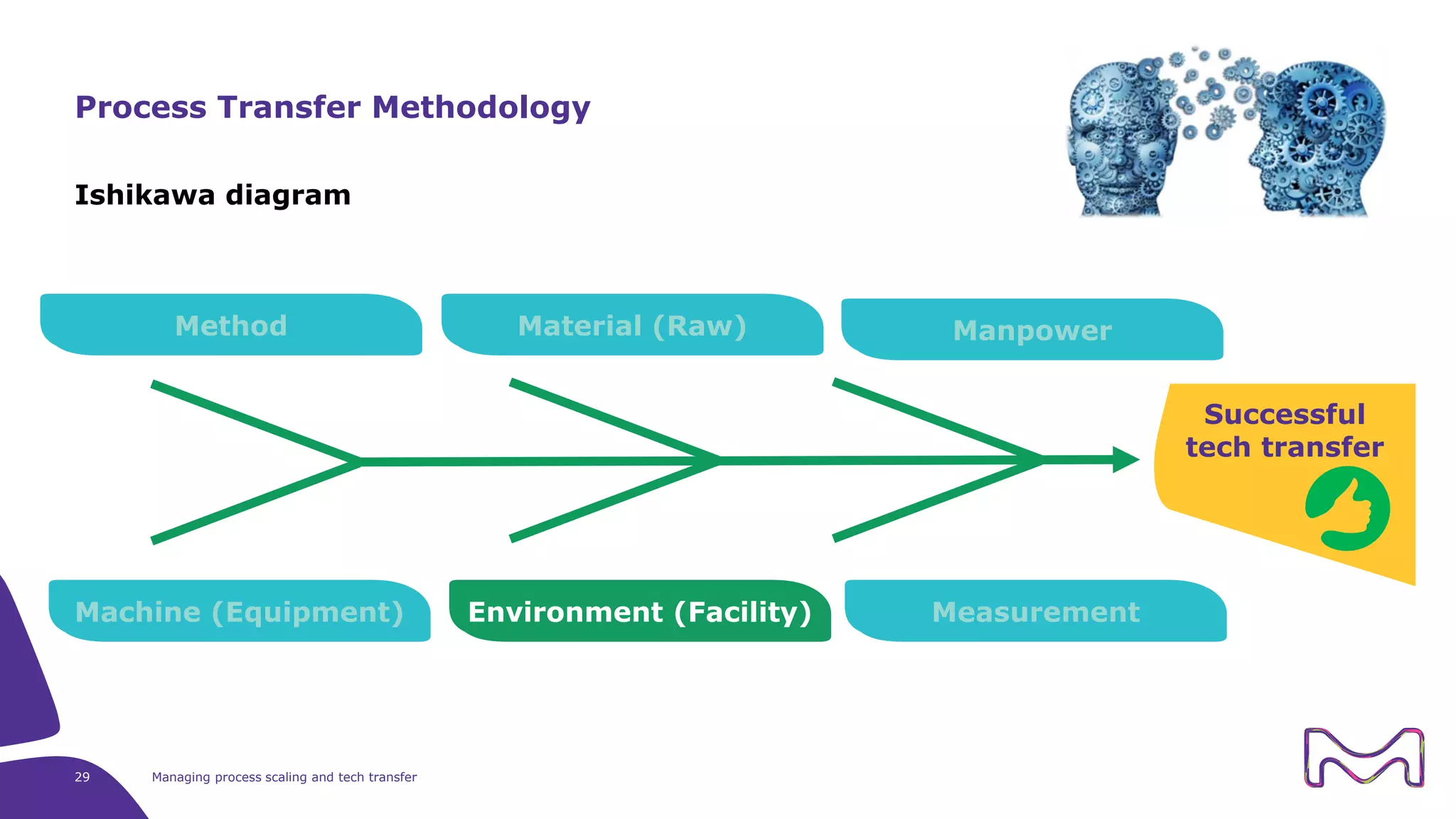
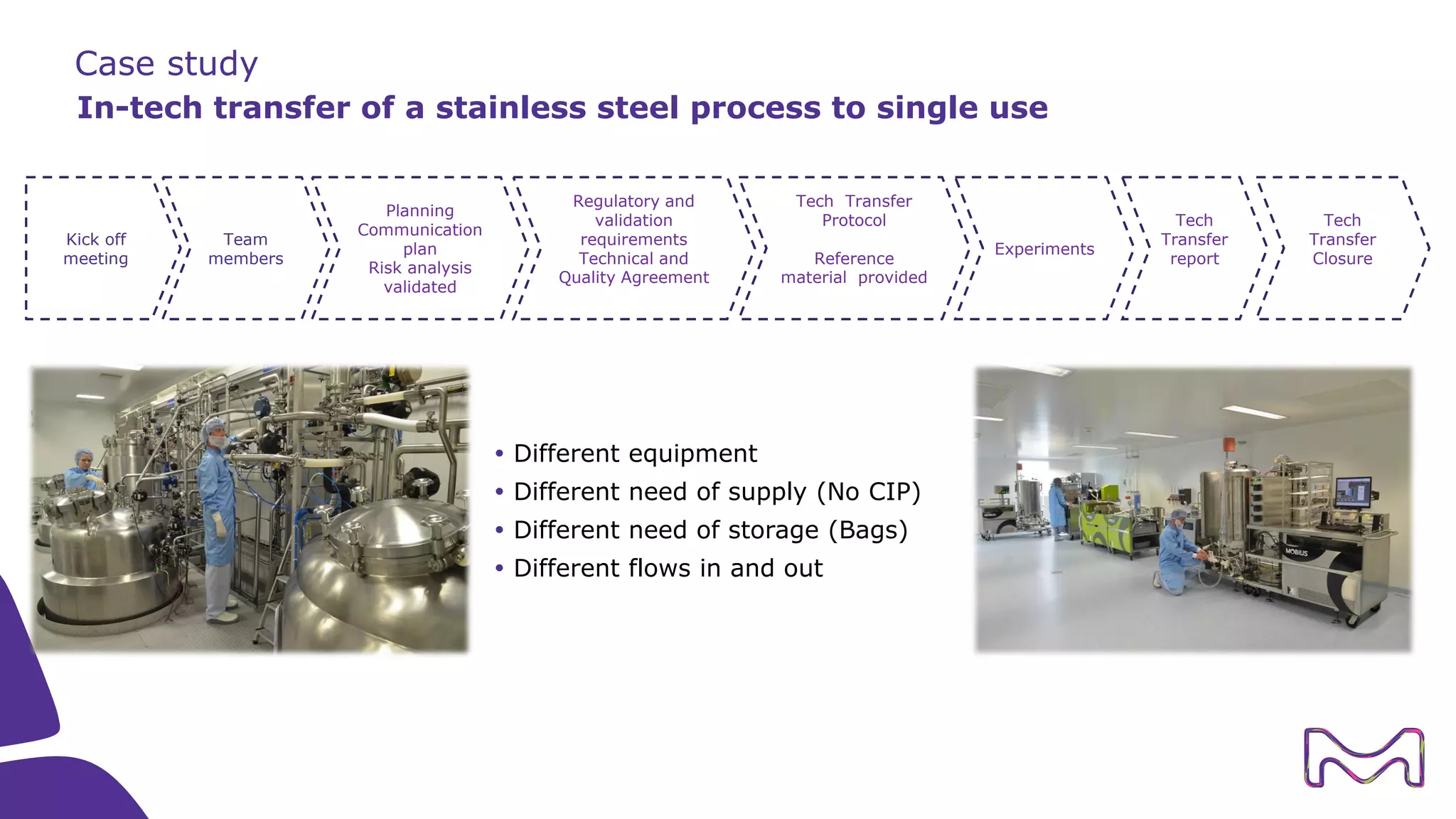
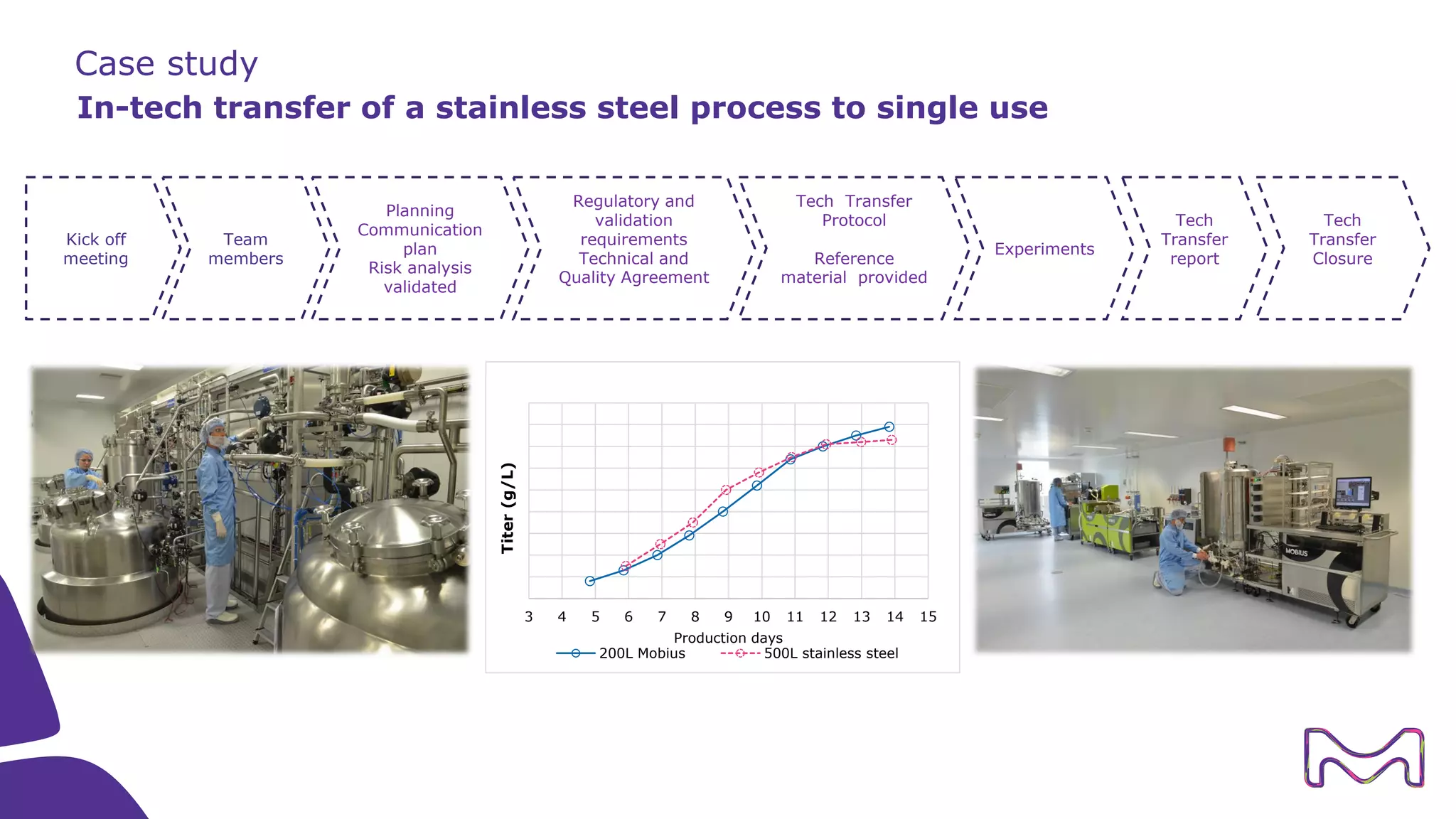
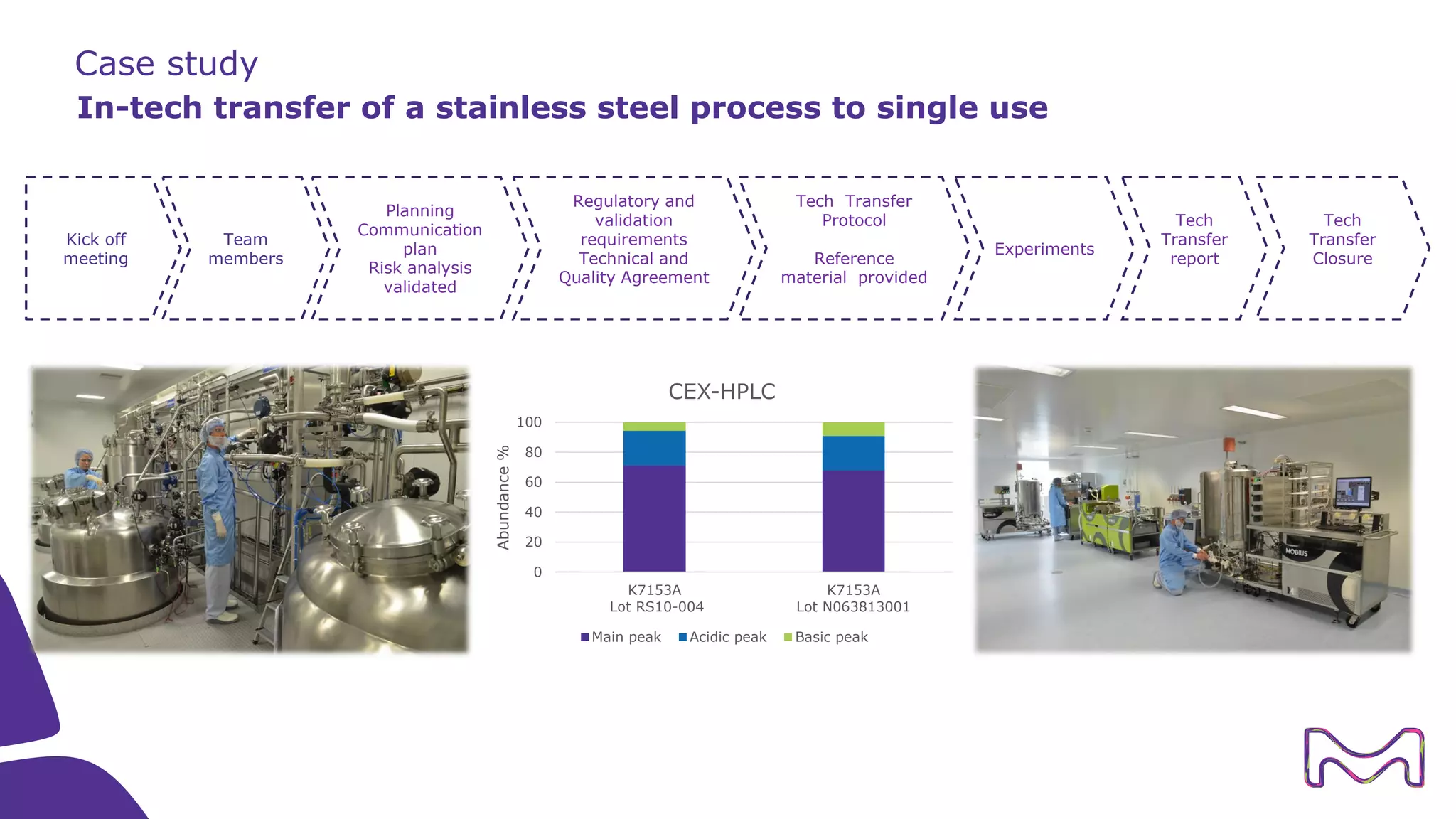
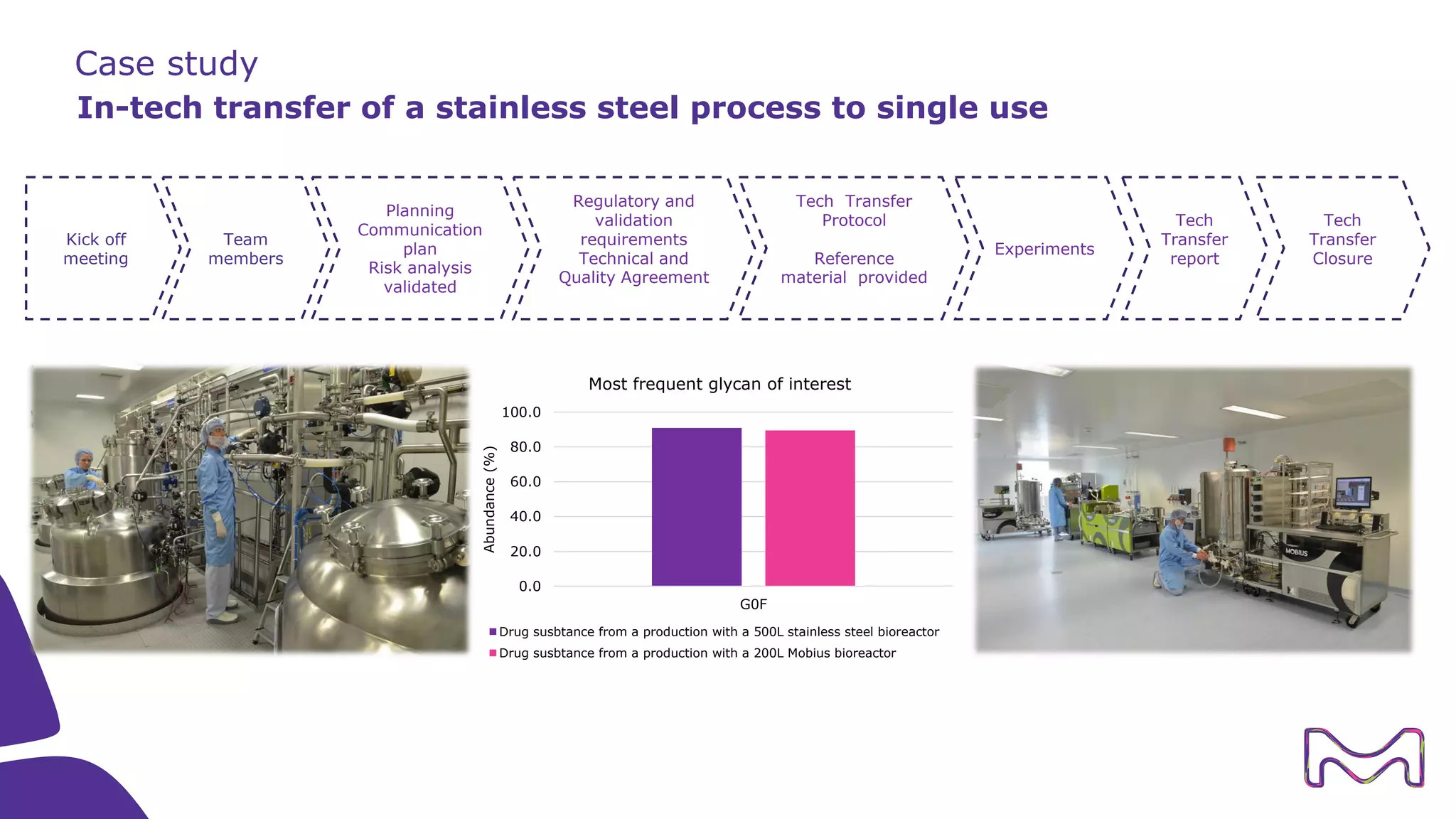
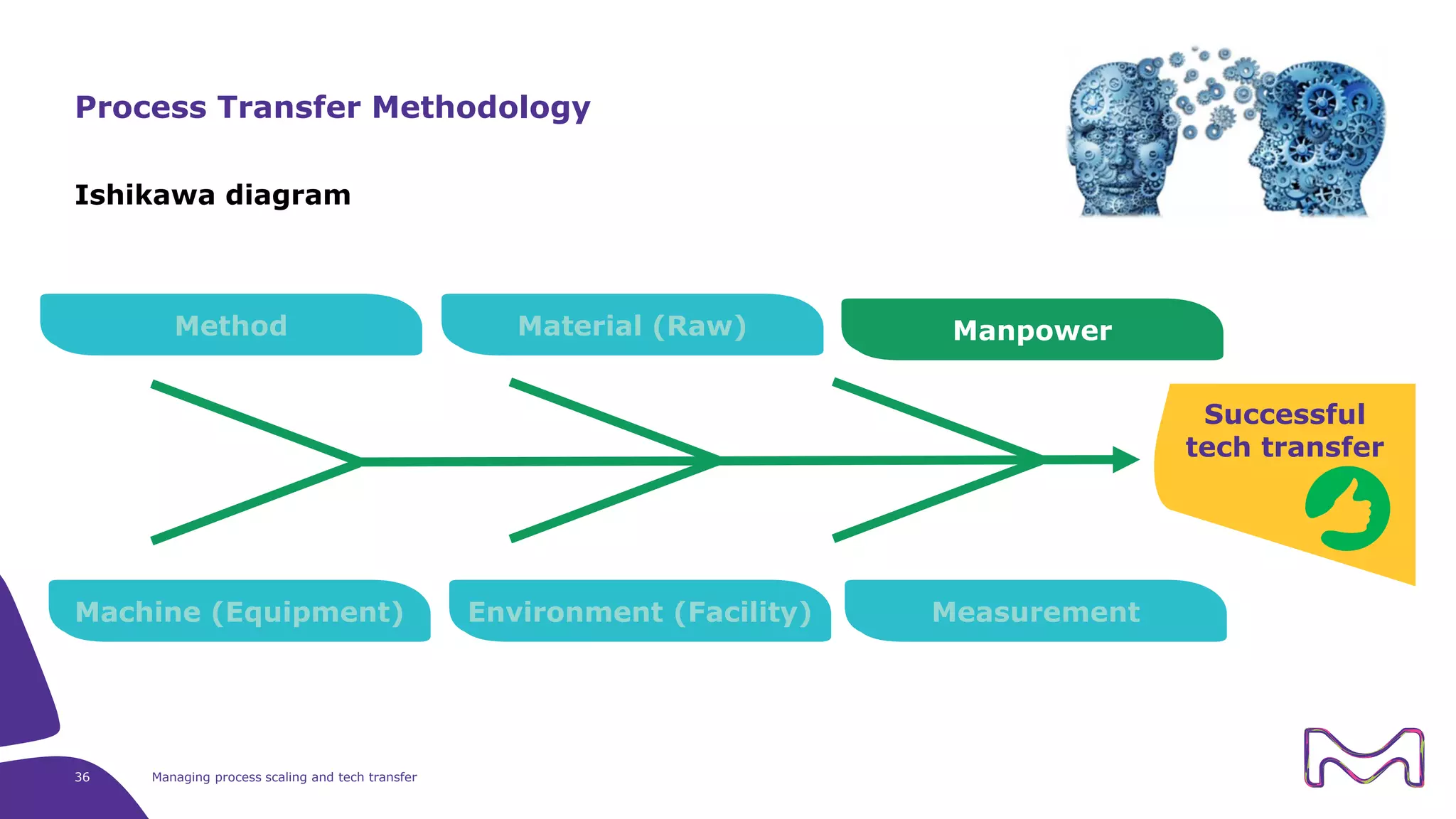
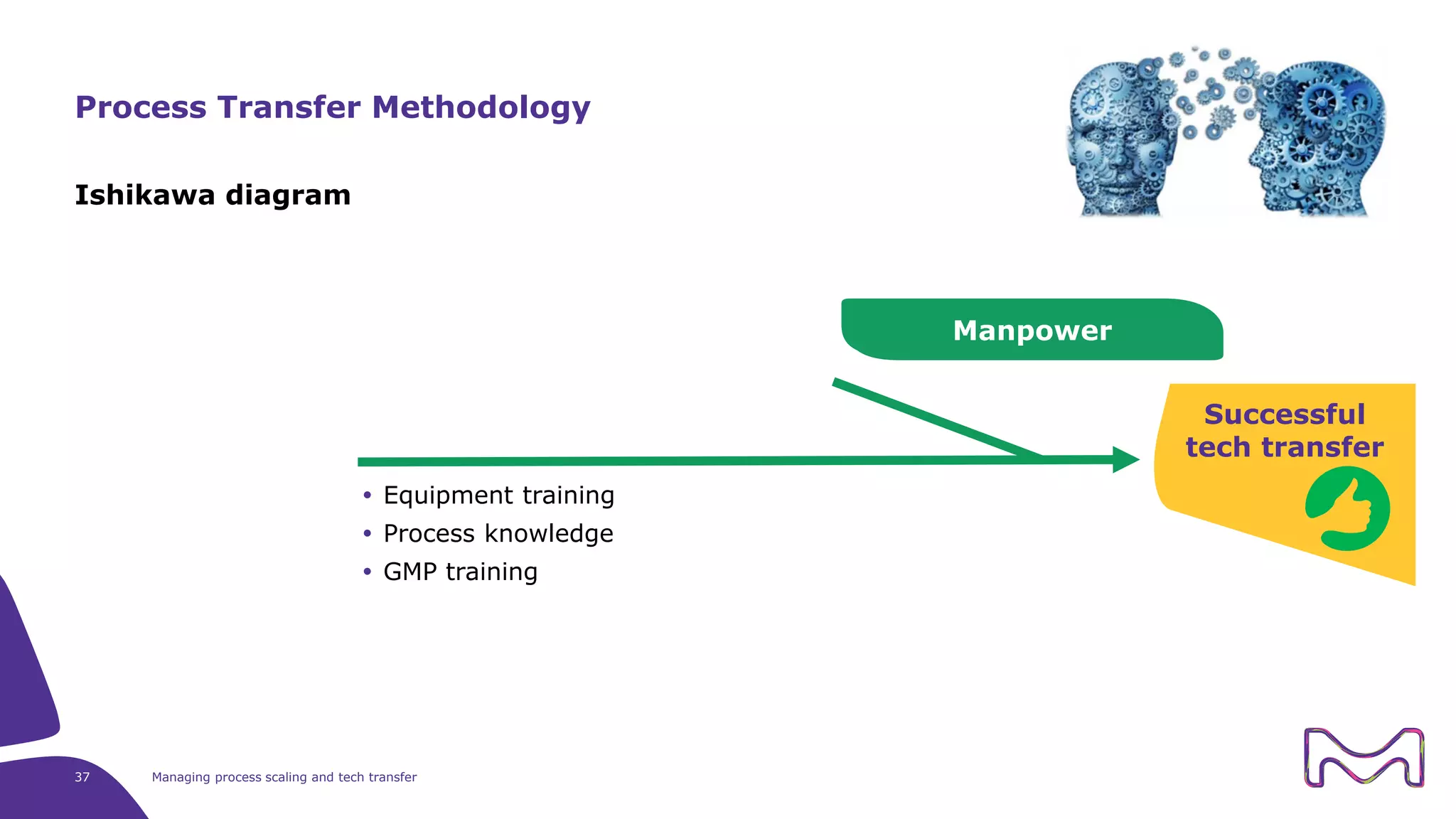
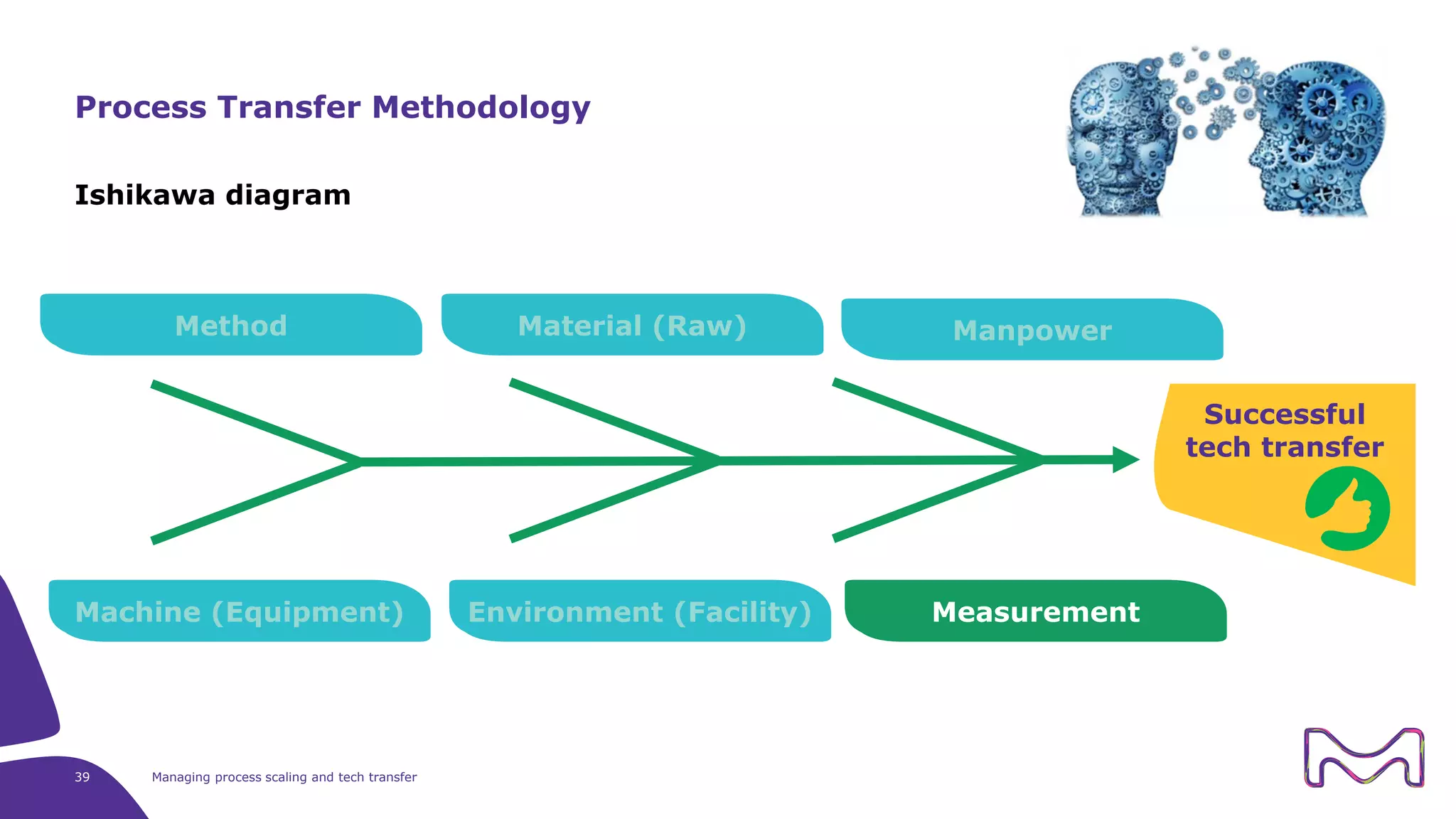
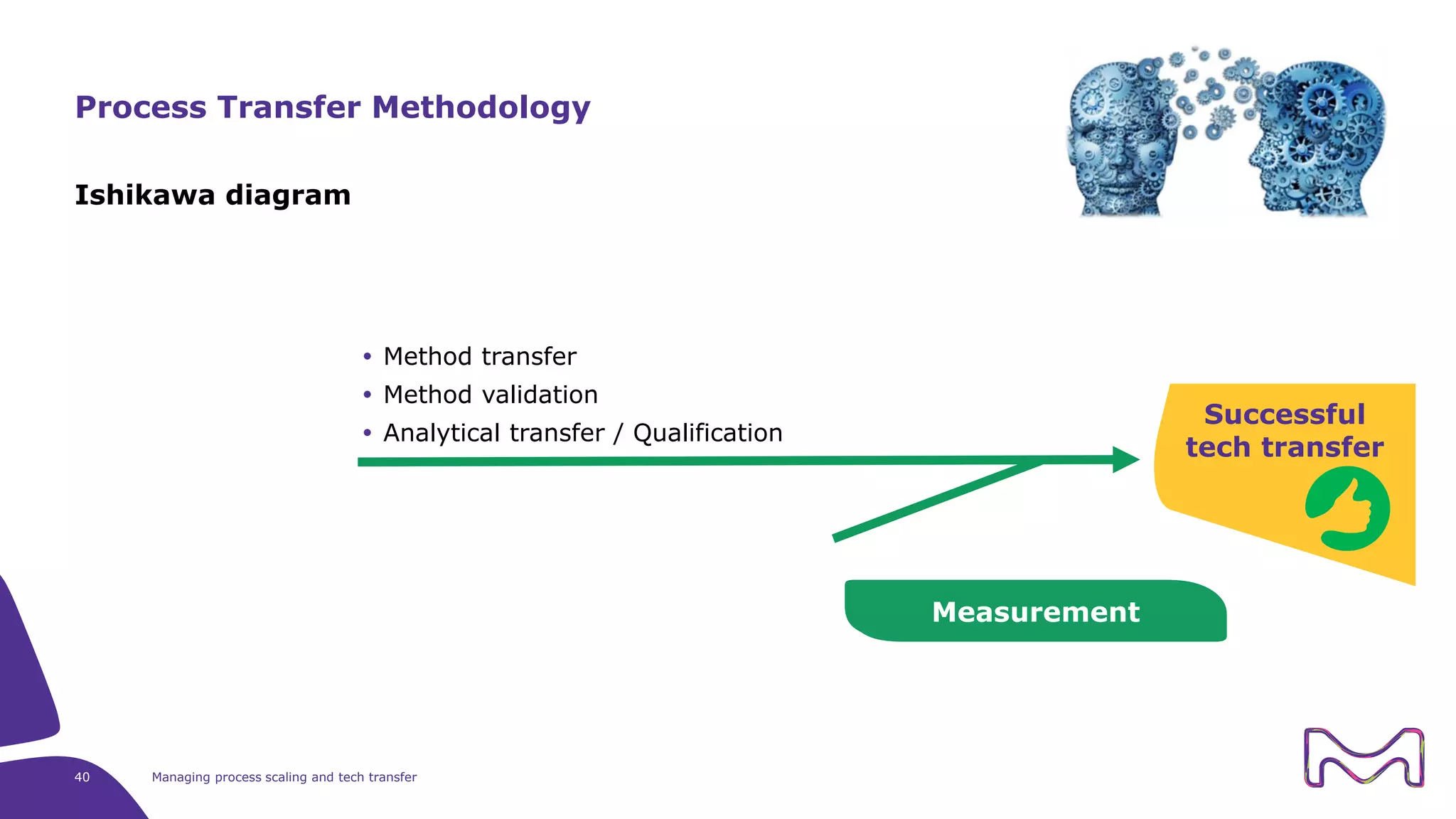
The document discusses the essential aspects of technology transfer and process scaling in biomanufacturing, emphasizing the importance of methodical organization and documentation throughout the transfer process. It outlines a comprehensive methodology for process changes, risk management, and equipment considerations, alongside detailed case studies on transferring stainless steel processes to single-use systems. Key activities include gap analysis, training, and ongoing support to ensure successful tech transfer and regulatory compliance.