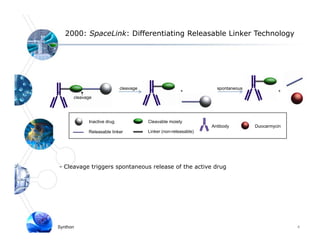
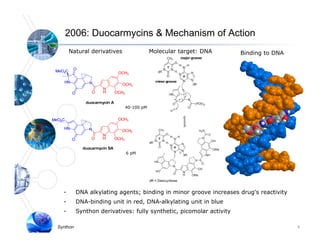
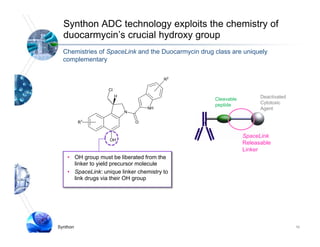
1) Syntarga was founded in 2001 to develop antibody-drug conjugate (ADC) technology using a novel releasable linker called SpaceLink.
2) In 2011, Syntarga was acquired by Synthon BV, a pharmaceutical company focused on biosimilars, monoclonal antibodies, and ADCs for oncology and multiple sclerosis.
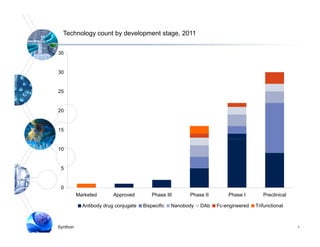
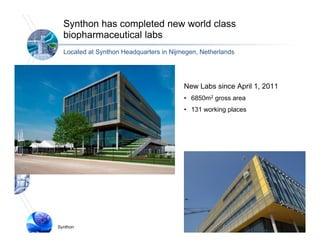
3) Synthon has advanced Syntarga's ADC technology, completing new biopharmaceutical laboratories and developing optimized linker-drug combinations that show potent anti-tumor activity in preclinical models of cancer.