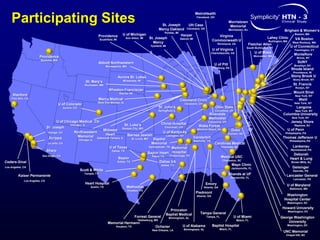
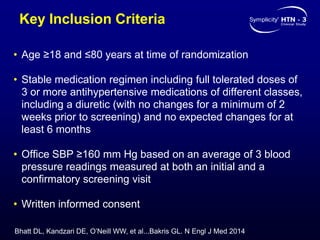
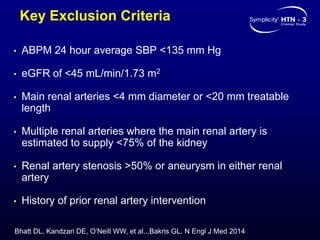
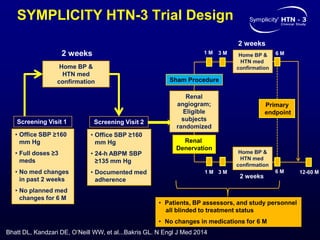
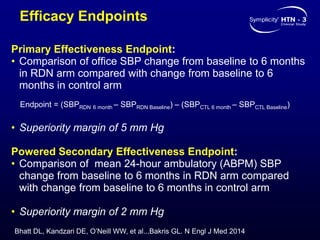
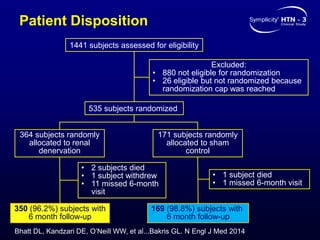
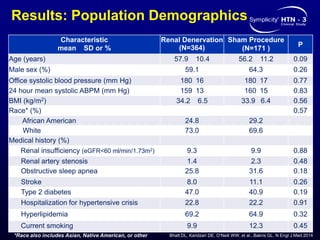
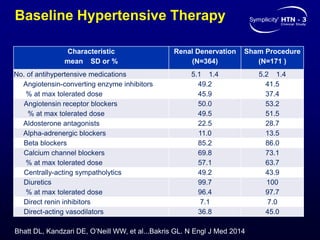
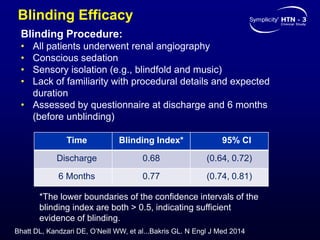
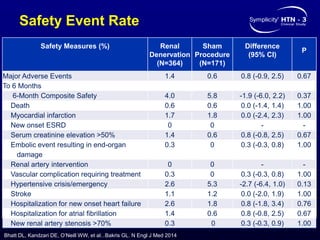
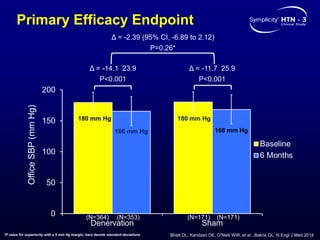
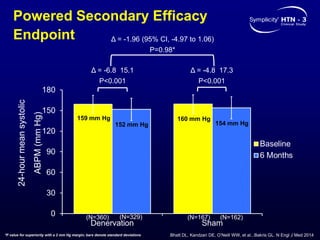
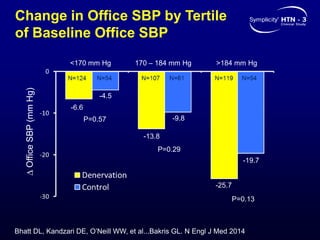
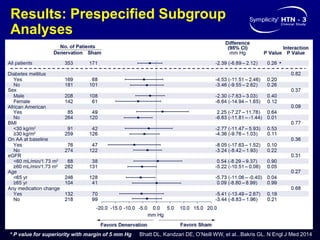
This randomized controlled trial evaluated the safety and efficacy of renal denervation for treatment-resistant hypertension. 535 patients with uncontrolled hypertension despite receiving 3 or more antihypertensive medications were randomized to renal denervation or a sham procedure. The primary outcome was the difference in office systolic blood pressure reduction between groups at 6 months, with a non-inferiority margin of 5 mmHg. Renal denervation did not meet the primary endpoint, with a between-group difference of -2.39 mmHg (p=0.26). It also did not meet the powered secondary endpoint of 24-hour ambulatory blood pressure change with a margin of 2 mmHg. Major adverse event rates were similar between groups