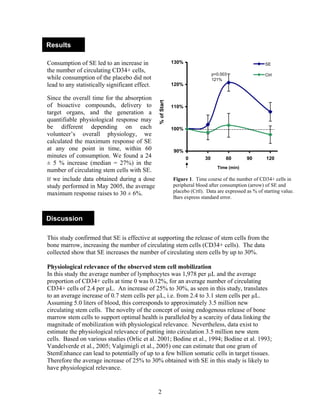
A double-blind, randomized, placebo-controlled study found that consumption of StemEnhance (SE) led to a 24-30% increase in circulating stem cells compared to placebo. SE significantly increased the number of CD34+ stem cells in blood samples taken 30-120 minutes after ingestion. This increase corresponded to approximately 3.5 million new circulating stem cells in the bloodstream, which studies suggest could result in billions of new cells in target tissues and may have physiological relevance.