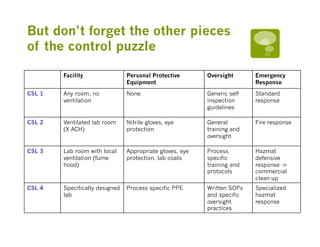
This document discusses the development and use of chemical safety levels (CSLs) for laboratory risk assessment. It argues that CSLs could help address evolving challenges in laboratory safety by providing a framework to assess risk based on chemical hazards and select appropriate controls. The document outlines stakeholders in laboratory risk assessment and factors to consider when selecting a CSL level. It proposes conceptual CSL levels from 1 to 4 based on fire, corrosivity, reactivity and toxicity hazards and matching controls. Next steps include completing a risk assessment tool and guidance documents for implementing CSLs.