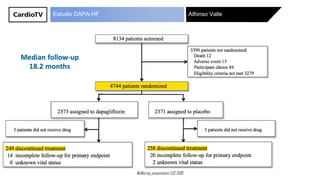
The DAPA-HF study presented by Alfonso Valle McMurray at ESC 2019 evaluated the effects of dapagliflozin in patients with heart failure and reduced ejection fraction. Results indicated that the drug reduced the composite outcome of cardiovascular death or heart failure exacerbation by 26%, decreased the risk of first heart failure hospitalization by 30%, and showed significant improvements in patient-reported outcomes. Additionally, there was a nominally significant 17% reduction in all-cause mortality.








![Alfonso Valle
McMurray presentation ESC 2019.
1.Time to the first occurrence of either of the components of the composite: CV death or hospitalization for HF.
2.Total number of (first and recurrent) HF hospitalizations and CV death
3.Change from baseline measured at 8 months in the total symptom score of the Kansas City Cardiomyopathy Questionnaire (KCCQ), a specific HF
patient reported outcome questionnaire.
4.Time to the first occurrence of any of the components of the composite: ≥50% sustained decline in eGFR or reaching End Stage Renal Disease
(ESRD) or renal death. [ Time Frame: From randomization visit (day 0) up to approximately 3 years ]End Stage Renal Disease (ESRD) is defined as
-Sustained eGFR <15 mL/min/1.73m^2 or,
-Chronic dialysis treatment or,
-Receiving a renal transplant
5. Time to death from any cause.
Estudio DAPA-HF](https://image.slidesharecdn.com/4-190901181729/85/DAPA-HF-Trial-10-320.jpg)






