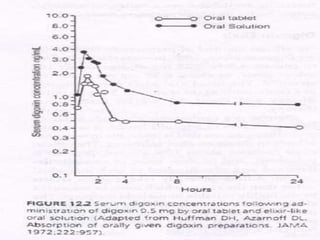
This document discusses various types of solutions used in pharmaceutical preparations. It defines solutions as liquid preparations containing one or more substances dissolved in a suitable solvent. Solutions can be classified based on their use, such as oral, ophthalmic, or topical solutions. Common solvents used in pharmaceutical preparations include water, alcohol, glycerin, propylene glycol, and various oils. Key factors that determine solubility of substances, such as temperature, pH, and salt forms, are also covered.