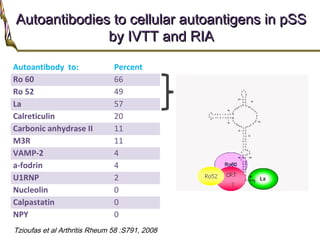
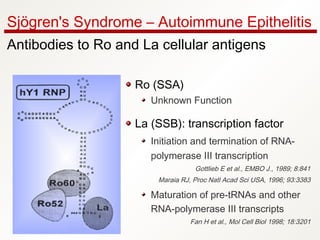
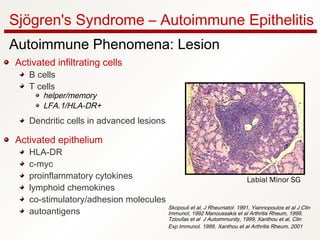
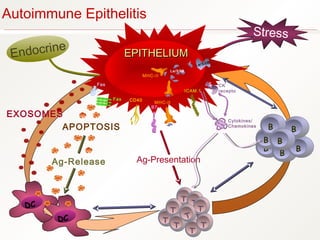
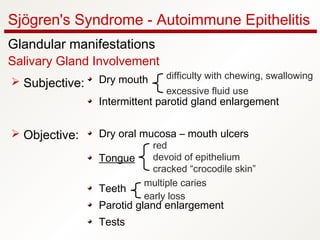
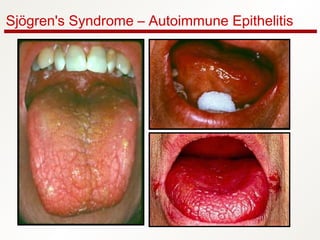
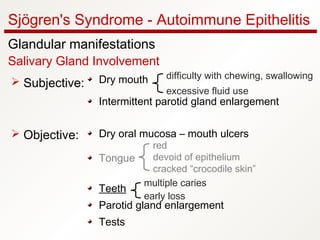
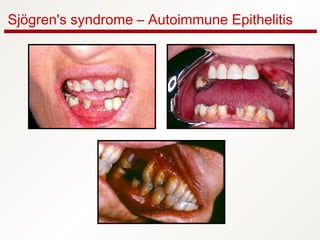
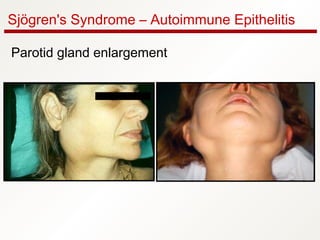
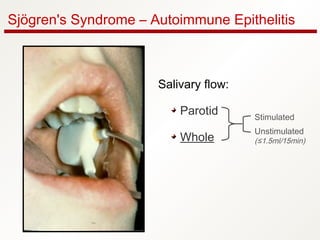
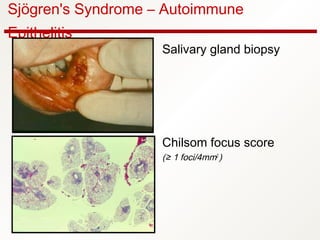
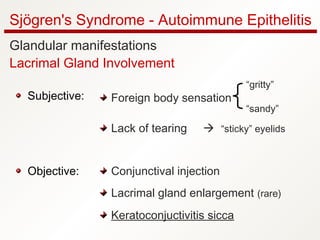
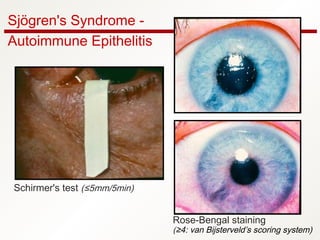
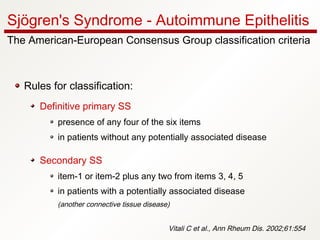
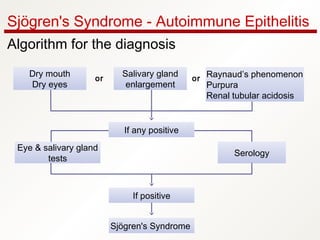
1. Sjögren's syndrome is an autoimmune disease that primarily affects the lacrimal and salivary glands, causing dry eyes and dry mouth.
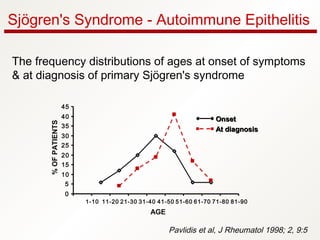
2. It most commonly affects women in the fourth to fifth decade of life, with a female to male ratio of approximately 9:1.
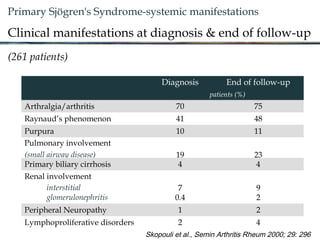
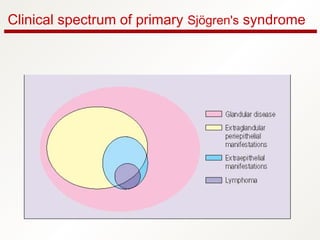
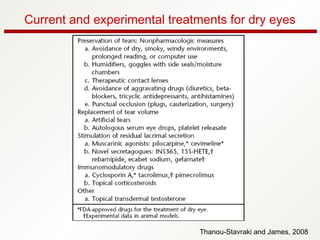
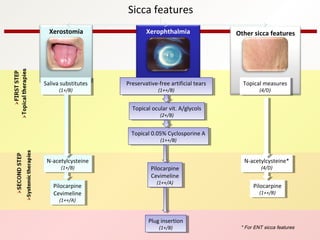
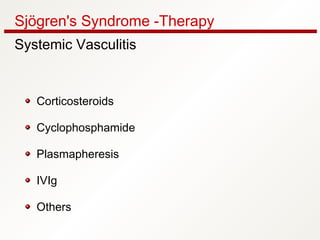
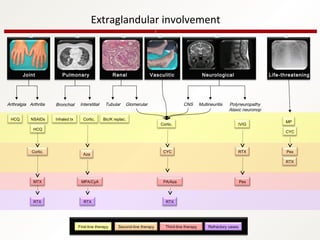
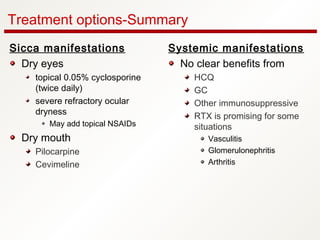
3. The disease can range from purely glandular involvement to systemic features affecting organs like the lungs, kidneys, liver, blood vessels, and nerves. Treatment focuses on managing sicca symptoms as well as systemic manifestations.