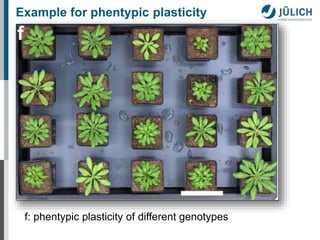
The document summarizes research on plant phenotyping conducted at the Forschungszentrum Jülich. It describes phenotyping as quantifying plant traits in space and time, including effects of environment and genetics. Methods discussed include automated measurements of shoots and roots, field phenotyping using mini-plots and aerial sensors, and 3D reconstruction of canopies. Examples demonstrate quantifying photosynthesis and measuring various plant traits from airborne platforms to better understand crop responses and gene-environment interactions.