This document provides an overview of an ophthalmology textbook. It includes information about the authors, contributors, publisher, and copyright details. The preface written by the lead author, Gerhard K. Lang, explains the educational goals behind creating the textbook - to engage medical students and sustain their interest in ophthalmology. A table of contents provides a high-level outline of the textbook's chapter structure and topics covered.

![II
Library of Congress Cataloging-in-Publica-
tion Data
Lang, Gerhard K.
[Augenheilkunde. English]
Ophthalmology : a short textbook /
Gerhard K. Lang ; with contributions by
J. Amann... [et al.]. p. ; cm. Includes biblio-
graphical references and index.
ISBN 3131261617
1. Eye-Diseases. 2. Ophthalmology.
I. Amann, J. (Josef) II. Title.
[DNLM: 1. Eye Diseases.
WW 40 L269a 2000a]
RE46.L3413 2000
617.7–dc21 00-032597
Student contributors:
Christopher Dedner, Tübingen
Uta Eichler, Karlsruhe
Heidi Janeczek, Göttingen
Beate Jentzen, Husberg
Mathis Kayser, Freiburg
Kerstin Lipka, Kiel
Maren Molkewehrum, Kiel
Alexandra Ogilvie, Munich
Patricia Ogilvie, Würzburg
Stefan Rose, Oldenburg
Translated by John Grossman, Berlin,
Germany
This book is an authorized translation of the
German edition published and copyrighted
1998 by Georg Thieme Verlag, Stuttgart,
Germany.
Drawings by Markus Voll, Fürstenfeldbruck
Important Note: Medicine is an ever-
changing science undergoing continual
development. Research and clinical
experience are continually expanding our
knowledge, in particular our knowledge of
proper treatment and drug therapy. Insofar
as this book mentions any dosage or appli-
cation, readers may rest assured that the
authors, editors, and publishers have made
every effort to ensure that such references
are in accordance with the state of knowl-
edge at the time of production of the book.
Nevertheless this does not involve,
imply, or express any guarantee or
responsibility on the part of the publishers
in respect of any dosage instructions and
forms of application stated in the book.
Every user is requested to examine care-
fully the manufacturers’ leaflets accom-
panying each drug and to check, if neces-
sary in consultation with a physician or
specialist, whether the dosage schedules
mentioned therein or the contraindications
stated by the manufacturers differ from the
statements made in the present book. Such
examination is particularly important with
drugs that are either rarely used or have
been newly released on the market. Every
dosage schedule or every form of applica-
tion used is entirely at the user’s own risk
and responsibility. The authors and pub-
lishers request every user to report to the
publishers any discrepancies or inaccura-
cies noticed.
! 2000 Georg Thieme Verlag
Rüdigerstraße 14
D-70469 Stuttgart, Germany
Thieme New York, 333 Seventh Avenue
New York, N. Y. 10001 U.S.A
Typesetting by Druckhaus Götz GmbH,
Ludwigsburg
Printed in Germany by
Appl, Wemding
ISBN 3-13-126161-7 (GTV)
ISBN 0-86577-936-8 (TNY) 1 2 3 4 5
Some of the product names, patents, and
registered designs referred to in this book
are in fact registered trademarks or pro-
prietary names even though specific refer-
ence to this fact is not always made in the
text. Therefore, the appearance of a name
without designation as proprietary is not to
be construed as a representation by the
publisher that it is in the public domain.
This book, including all parts thereof, is
legally protected by copyright. Any use,
exploitation, or commercialization outside
the narrow limits set by copyright legisla-
tion, without the publisher’s consent, is
illegal and liable to prosecution. This
applies in particular to photostat reproduc-
tion, copying, mimeographing or duplica-
tion of any kind, translating, preparation of
microfilms, and electronic data processing
and storage.
Lang, Ophthalmology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.](https://image.slidesharecdn.com/shorttexetlangophthalmology2000thieme-230914204815-6096f09f/85/ShortTexet-Lang-Ophthalmology-2000-Thieme-pdf-2-320.jpg)








































































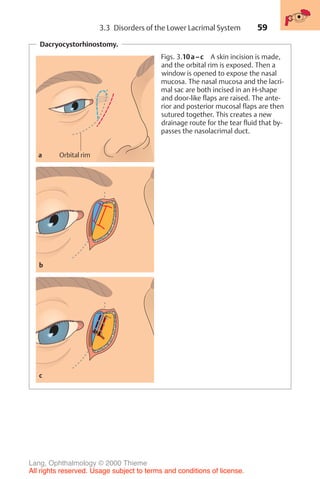




























































































































































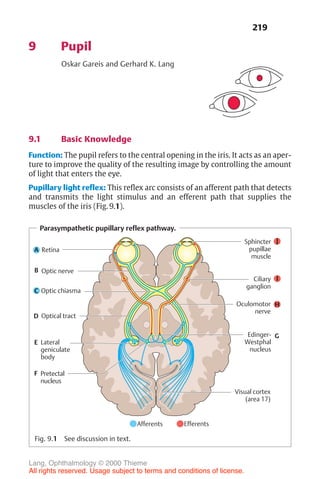













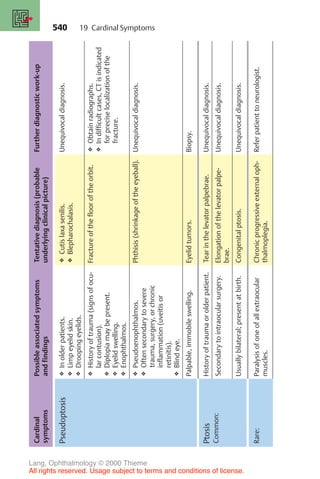
![233
10 Glaucoma
Gerhard K. Lang
10.1 Basic Knowledge
Definition
Glaucoma is a disorder in which increased intraocular pressure damages the
optic nerve. This eventually leads to blindness in the affected eye.
❖ Primary glaucoma refers to glaucoma that is not caused by other ocular
disorders.
❖ Secondary glaucoma may occur as the result of another ocular disorder or
an undesired side effect of medication or other therapy.
Epidemiology: Glaucoma is the second most frequent cause of blindness in
developing countries after diabetes mellitus. Fifteen to twenty per cent of all
blind persons lost their eyesight as a result of glaucoma. In Germany, approxi-
mately 10% of the population over 40 has increased intraocular pressure.
Approximately 10% of patients seen by ophthalmologists suffer from glau-
coma. Of the German population, 8 million persons are at risk of developing
glaucoma, 800 000 have already developed the disease (i.e., they have glau-
coma that has been diagnosed by an ophthalmologist), and 80 000 face the
risk of going blind if the glaucoma is not diagnosed and treated in time.
Early detection of glaucoma is one of the highest priorities for the pub-
lic health system.
Physiology and pathophysiology of aqueous humor circulation (Fig. 10.1):
The average normal intraocular pressure of 15 mm Hg in adults is significantly
higher than the average tissue pressure in almost every other organ in the
body. Such a high pressure is important for the optical imaging and helps to
ensure several things:
❖ Uniformly smooth curvature of the surface of the cornea.
❖ Constant distance between the cornea, lens, and retina.
❖ Uniform alignment of the photoreceptors of the retina and the pigmented
epithelium on Bruch’s membrane, which is normally taut and smooth.
The aqueous humor is formed by the ciliary processes and secreted into the
posterior chamber of the eye (Fig. 10.1 [A]). At a rate of about 2–6 µl per
Lang, Ophthalmology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.](https://image.slidesharecdn.com/shorttexetlangophthalmology2000thieme-230914204815-6096f09f/85/ShortTexet-Lang-Ophthalmology-2000-Thieme-pdf-251-320.jpg)
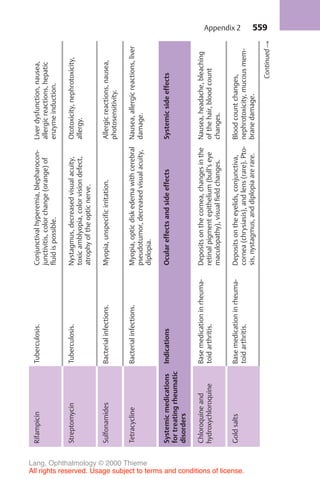
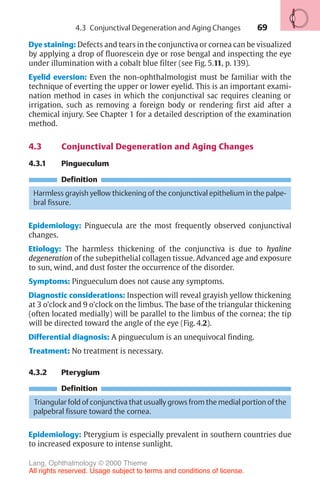
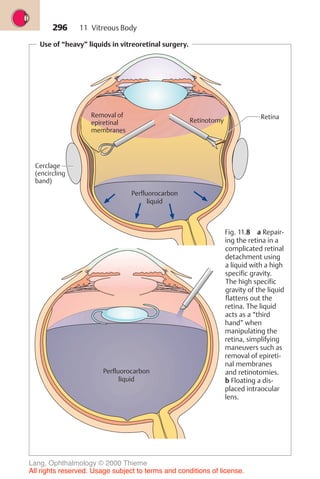
![234
Physiology of aqueous humor circulation.
A
E
D
C
B
Ciliary body
Lens
Iris
Cornea
Canal of Schlemm
Trabecular meshwork
Collecting channel
Conjunctiva
Episcleral venous plexus
Fig. 10.1 As it flows from the nonpigmented cells of the ciliary epithelia A to
beneath the conjunctiva D , the aqueous humor overcomes physiologic resistance
from two sources: the resistance of the pupil B and the resistance of the trabecular
meshwork C .
minute and a total anterior and posterior chamber volume of about
0.2–0.4 ml, about 1–2% of the aqueous humor is replaced each minute.
The aqueous humor passes through the pupil into the anterior chamber. As
the iris lies flat along the anterior surface of the lens, the aqueous humor can-
not overcome this pupillary resistance (first physiologic resistance; Fig. 10.1
[B]) until sufficient pressure has built up to lift the iris off the surface of the
lens. Therefore, the flow of the aqueous humor from the posterior chamber
into the anterior chamber is not continuous but pulsatile.
Any increase in the resistance to pupillary outflow (pupillary block) leads to
an increase in the pressure in the posterior chamber; the iris inflates anteri-
orly on its root like a sail and presses against the trabecular meshwork (Table
10.2). This is the pathogenesis of angle closure glaucoma.
Various factors can increase the resistance to pupillary outflow (Table
10.1). The aqueous humor flows out of the angle of the anterior chamber
through two channels:
❖ The trabecular meshwork (Fig. 10.1 [C]) receives about 85% of the out-
flow, which then drains into the canal of Schlemm. From here it is con-
10 Glaucoma
Lang, Ophthalmology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.](https://image.slidesharecdn.com/shorttexetlangophthalmology2000thieme-230914204815-6096f09f/85/ShortTexet-Lang-Ophthalmology-2000-Thieme-pdf-252-320.jpg)












































































































































































































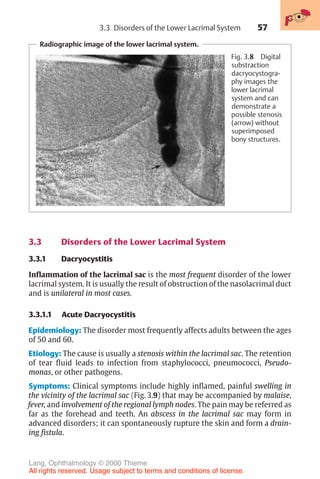
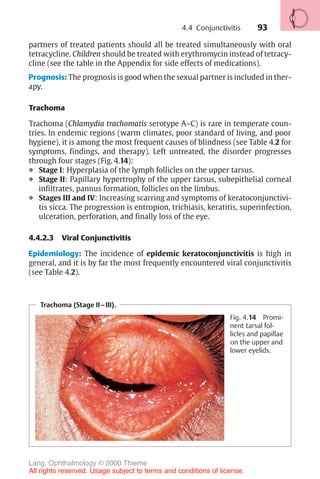
![443
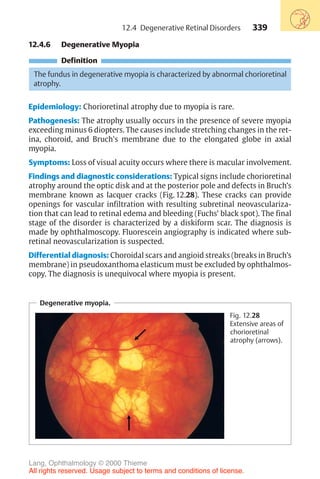
Diagnosis of corneal astigmatism with an ophthalmometer.
1 2
1 2
Fig. 16.14 The diagram shows the corneal reflex images (outline cross [1] and solid
cross [2]) of the Zeiss ophthalmometer. These images are projected on to the cor-
nea; the distance between them will vary depending on the curvature of the cornea.
The examiner must align the images by changing their angle of projection. After
aligning them, the examiner reads the axis of the main meridian, the corneal curva-
ture in millimeters, and the appropriate refractive power in diopters on a scale in the
device. This measurement is performed in both main meridians. The difference
yields the astigmatism. In irregular astigmatism, the images are distorted, and often
a measurement cannot be obtained.
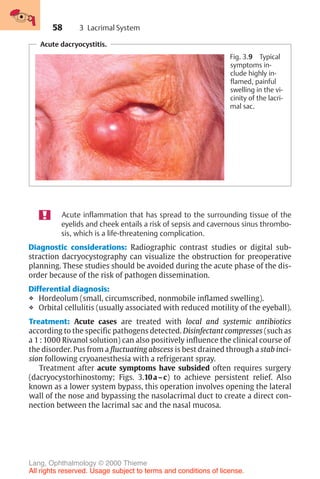
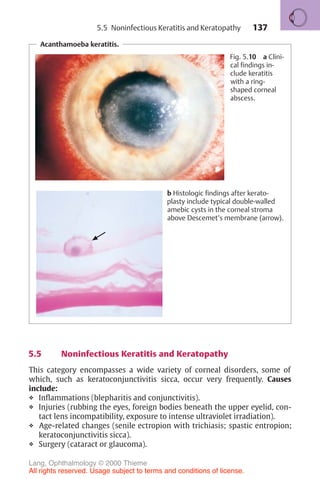
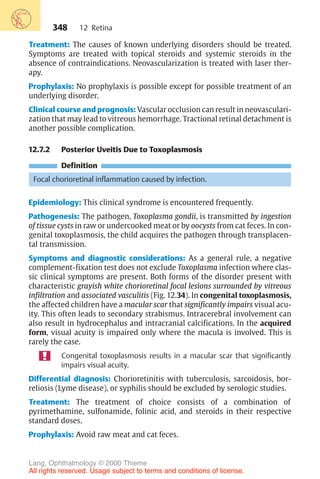
Correction of regular astigmatism with cylinder lenses.
0
0
90°
90°
180
180
a
b
c
d
Fig. 16.15 a Cyl-
inder lenses refract
light only in the
plane perpendicu-
lar to the axis of
the cylinder. The
axis of the cylinder
defines the nonre-
fracting plane.
b–d Cylinder
lenses can be man-
ufactured as plus
cylinders (c) or
minus cylinders
(d).
16.3 Refractive Anomalies
Lang, Ophthalmology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.](https://image.slidesharecdn.com/shorttexetlangophthalmology2000thieme-230914204815-6096f09f/85/ShortTexet-Lang-Ophthalmology-2000-Thieme-pdf-461-320.jpg)