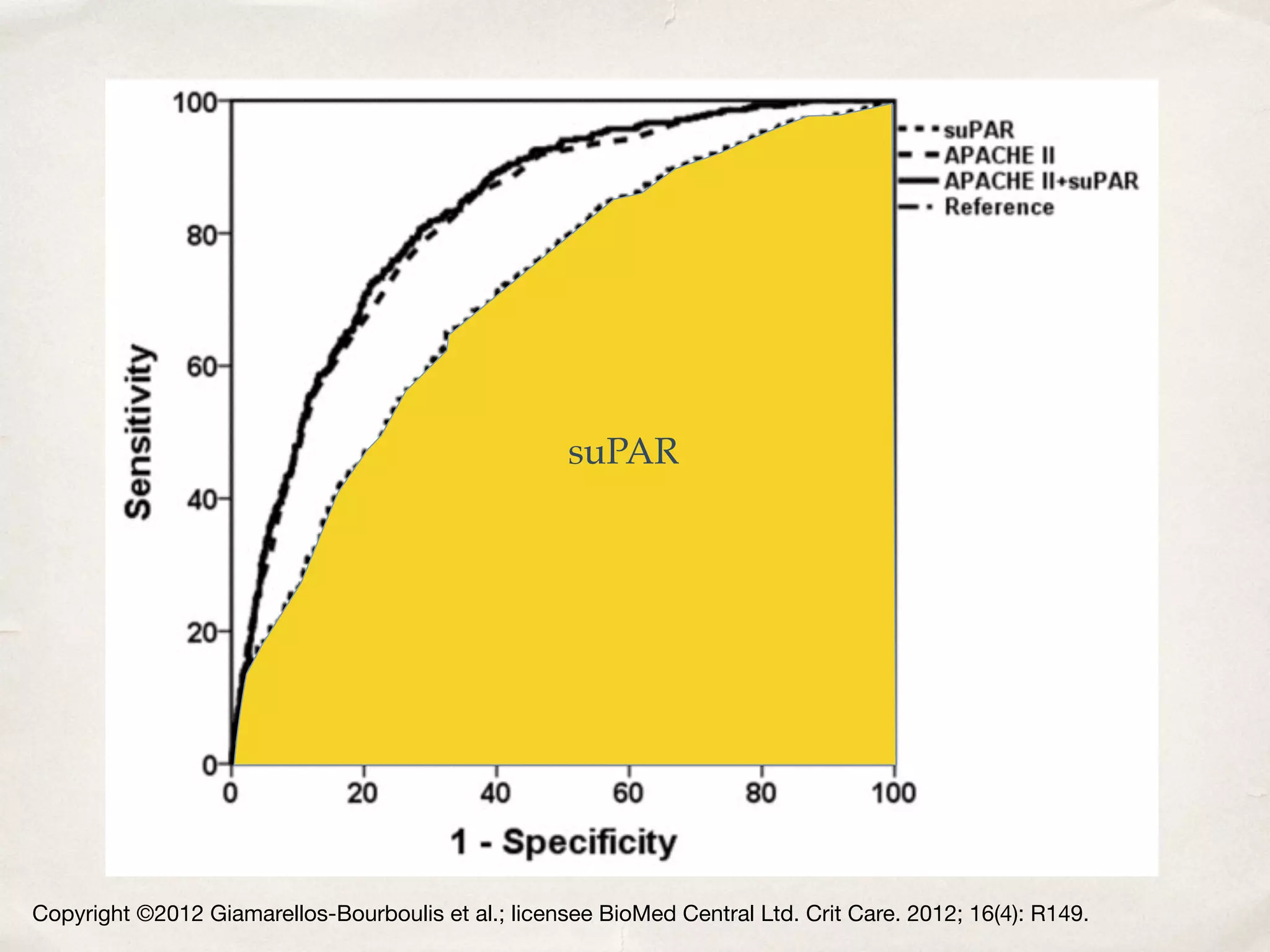
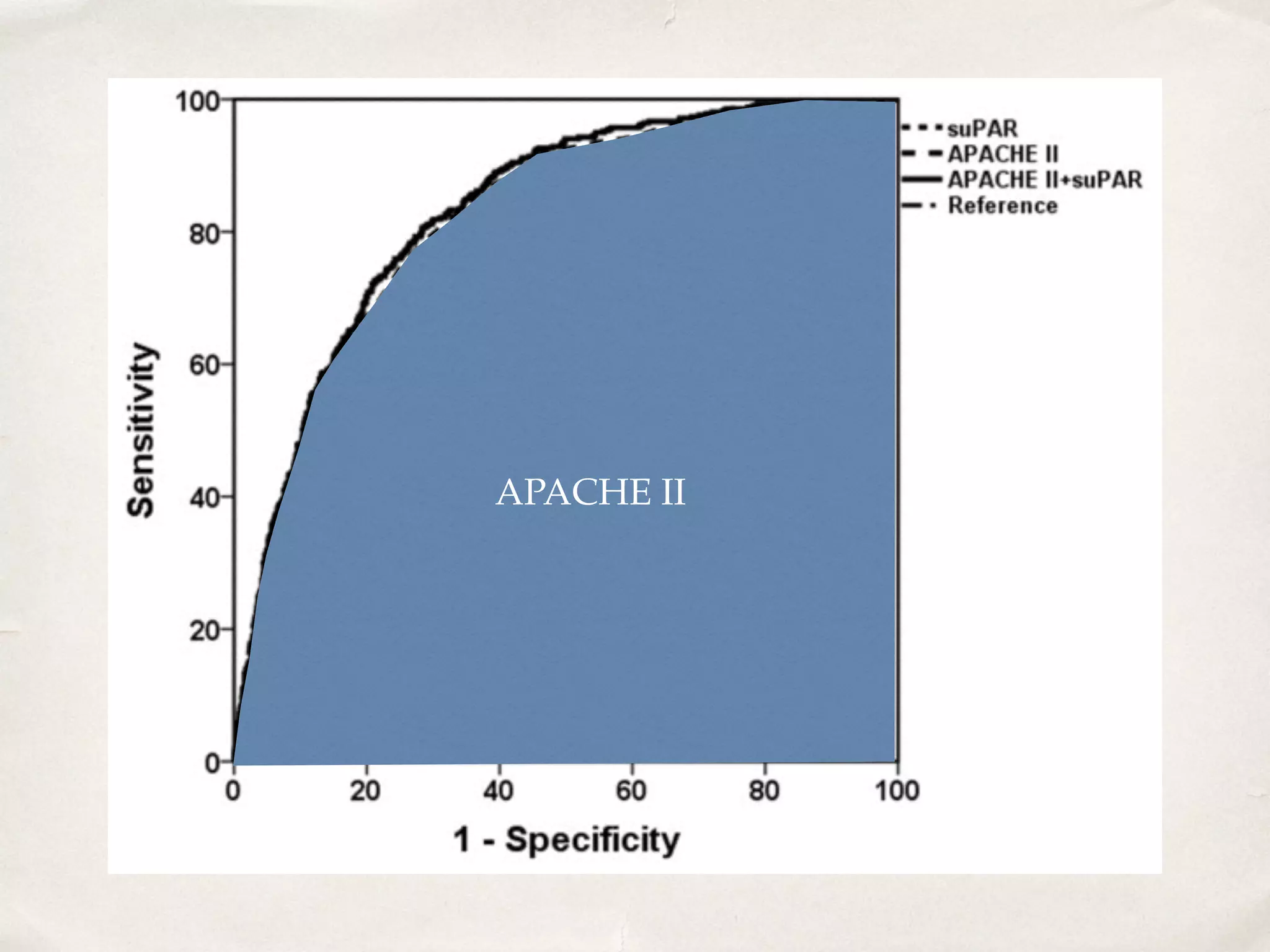
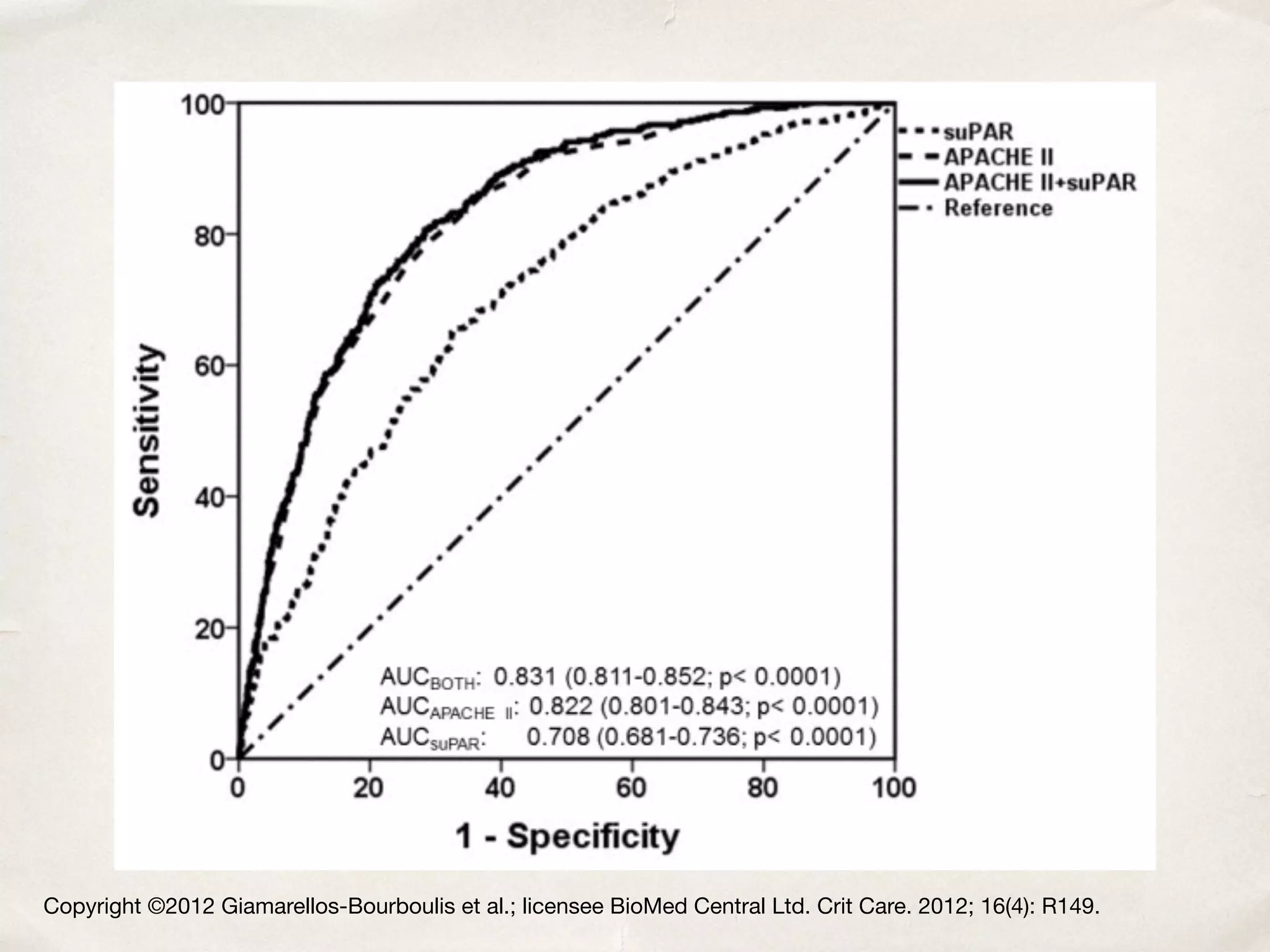
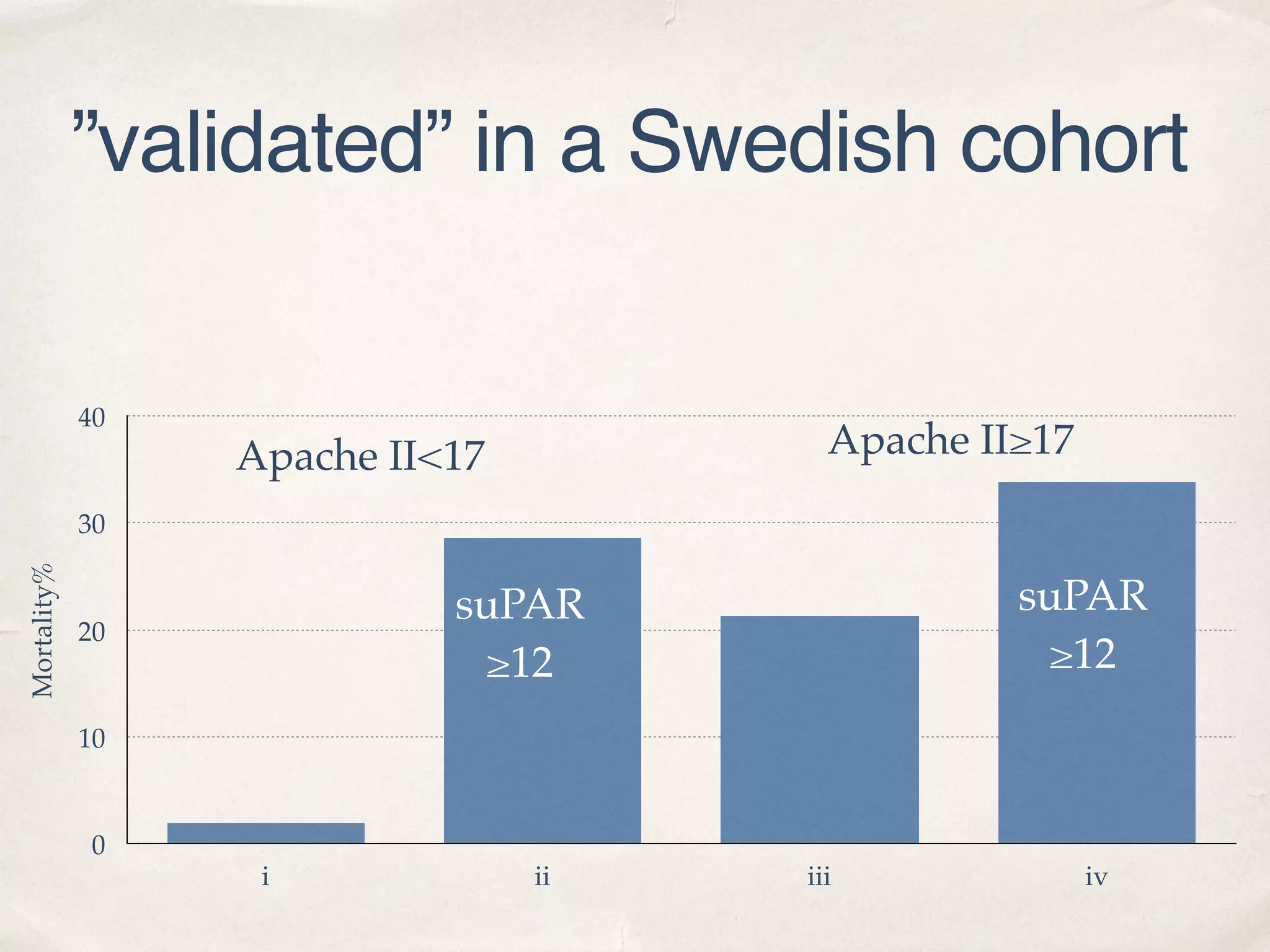
The document discusses a groundbreaking blood test using the biomarker supar, which can predict chronic kidney disease (CKD) five years before symptoms develop, potentially benefiting 15-25% of CKD patients with a sales potential exceeding $1 billion. It highlights findings from studies validating supar's strong prognostic value in intensive care unit (ICU) patients, though limited treatment options may restrict its lifesaving impact. Additional biomarkers are suggested to enhance predictive capabilities in clinical settings.