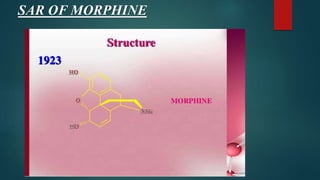
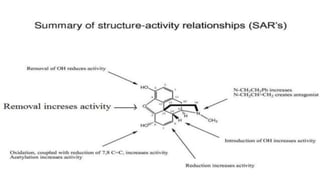
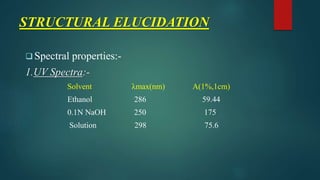
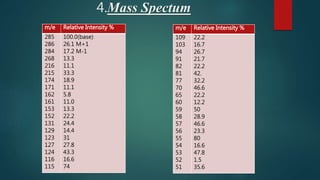
This document outlines the structural elucidation of morphine. Morphine is a colorless crystalline alkaloid derived from the opium poppy with analgesic and sedative effects. Spectral analysis was used to determine morphine's structure. UV/Vis spectroscopy showed absorption peaks characteristic of morphine. Infrared spectroscopy identified functional groups such as hydroxyl and aromatic groups. NMR spectroscopy assigned peaks for morphine's aromatic and methyl protons. Mass spectrometry analysis of morphine hydrochloride's fragmentation pattern yielded a molecular ion peak of m/e 285, confirming its molecular formula of C17H19NO3.