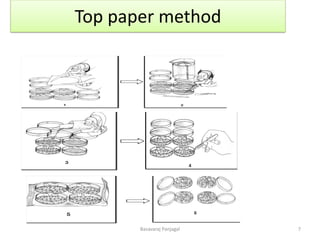
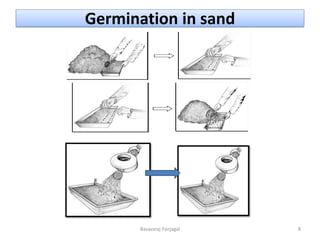
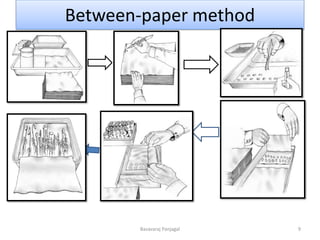
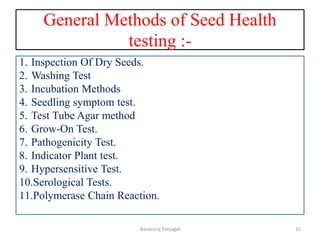
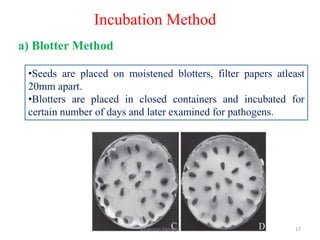
The document discusses various methods for seed health testing. It describes germination tests including the top-paper, between-paper, sand, and agar methods. Other tests discussed are the washing test, incubation methods using blotters or agar plates, seedling symptom tests, test tube agar methods, and grow-on tests. Objectives of seed health testing are to identify quality problems, determine planting value, and check for diseases. Specific methods covered in detail include the washing test, incubation methods, seedling symptom test, and test tube agar method.