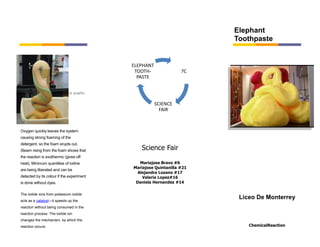
Elephant toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide when combined with dish soap and potassium iodide solution. This classroom demonstration produces a large amount of foam that "erupts" out of the graduated cylinder used in the reaction. Steam rises from the foam, indicating an exothermic reaction, and a small amount of iodine is released, detectable by its brown color in the foam. The potassium iodide acts as a catalyst to speed up the reaction without being consumed.