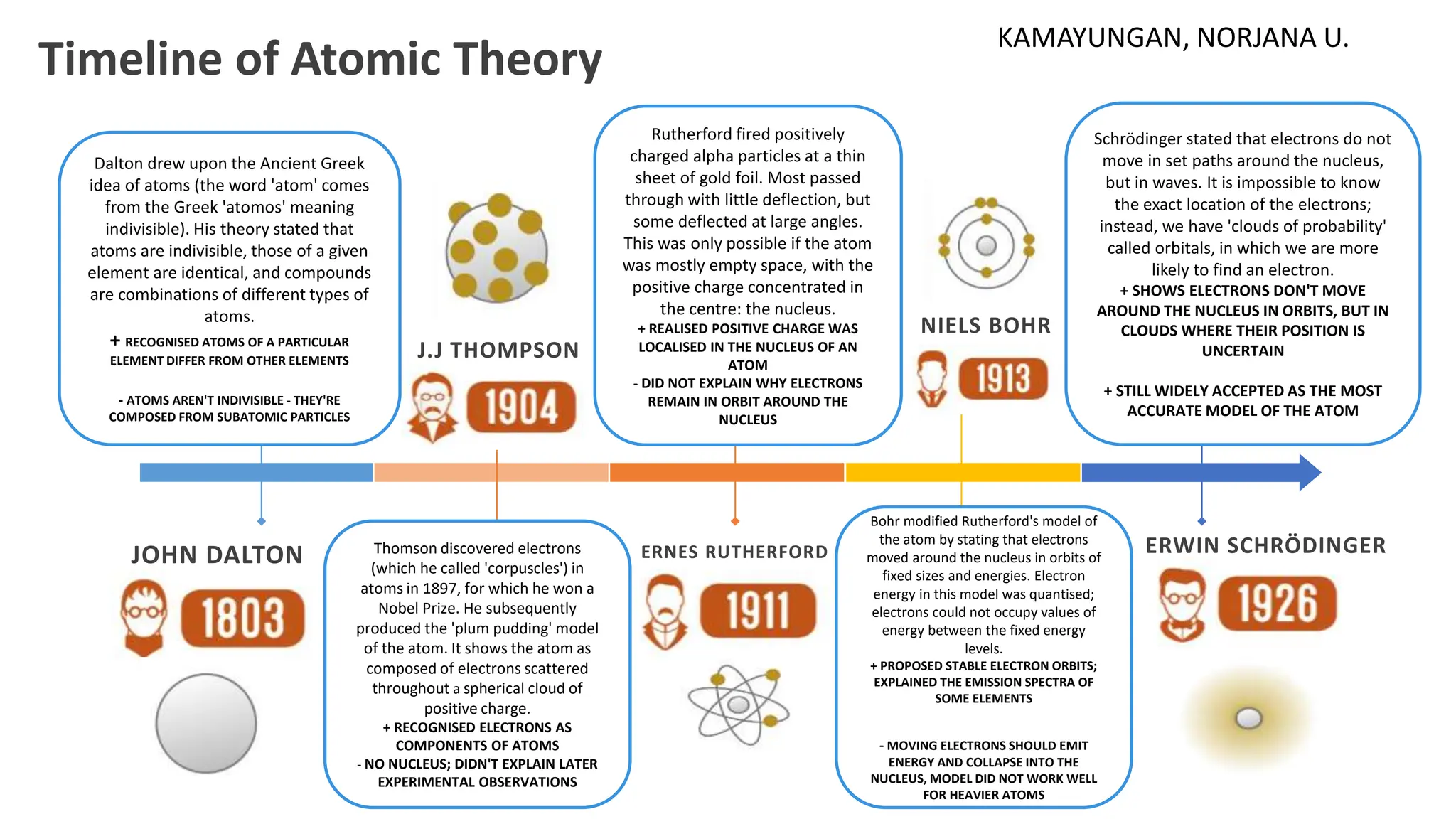
The document outlines the development of atomic theory from early ideas by John Dalton to modern concepts by Erwin Schrödinger. Dalton proposed that atoms are indivisible and identical within elements, while J.J. Thomson introduced electrons and the 'plum pudding' model. Rutherford discovered the nucleus, Bohr established fixed electron orbits, and Schrödinger described electrons as wave-like clouds, leading to the current understanding of atomic structure.