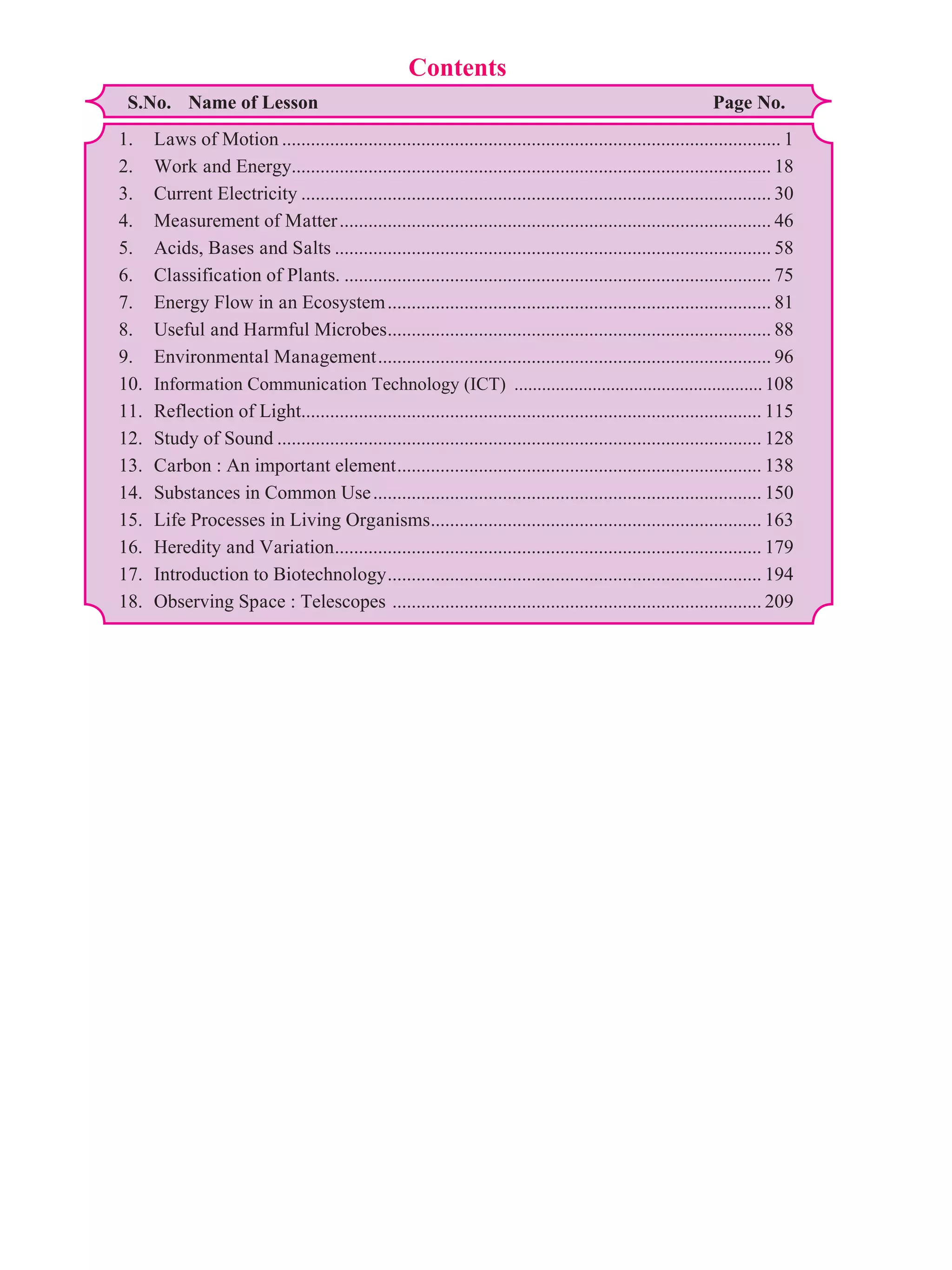
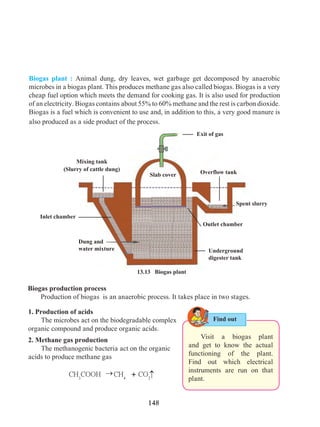
This document contains a table of contents for a science textbook. It lists 18 chapters with their corresponding page numbers, covering topics like laws of motion, work and energy, electricity, plants, ecosystems, light, and more. The document also provides some sample text from the first chapter on laws of motion, which discusses concepts like displacement, distance, speed, velocity, uniform and non-uniform motion, and acceleration. Examples are given to illustrate these concepts.




![8
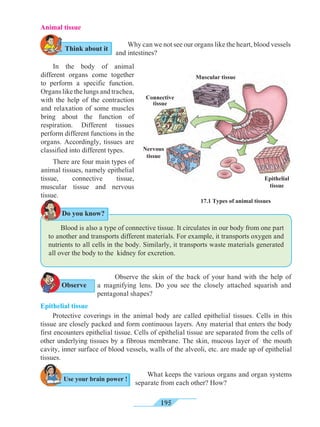
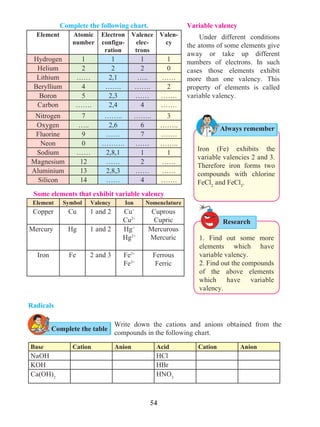
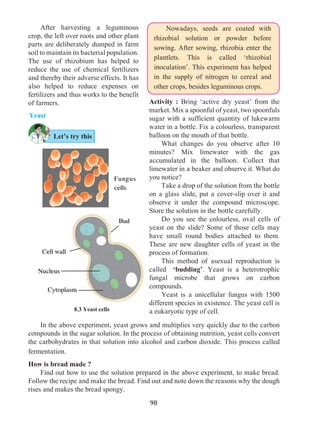
Equation describing the relation between displacement and time
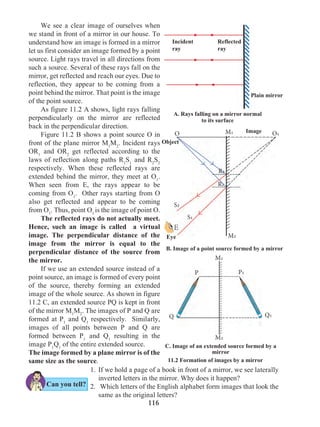
Let us suppose that an object in uniform acceleration ‘a’ and it has covered the
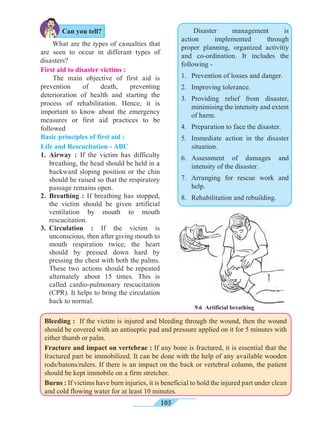
distance ‘s’ within time ‘t’. From the graph in figure 1.9, the distance covered by the object
during time ‘t’ is given by the area of quadrangle DOEB.
s = area of quadrangle DOEB
= area (rectangle DOEA) + area of triangle (DAB)
s = (AE × OE ) + ( × [AB × DA])
1
2
Equation describing the relation between velocity and time
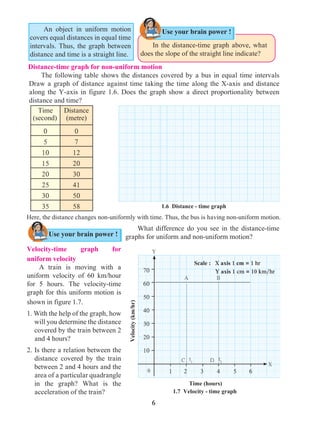
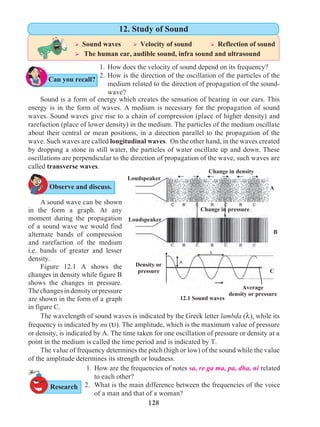
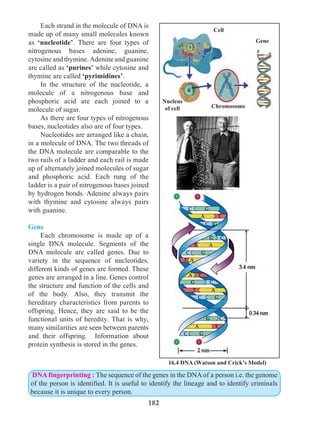
Figure 1.9 shows the change in velocity with time of a uniformly accelerated object.
The object starts from the point D in the graph with velocity u. Its velocity keeps increasing
and after time t, it reaches the point B on the graph.
v = u + at This is the relation between velocity and time.
s = ut + at2
This is the relation between displacement and time.
v2
= u2
+ 2as This is the relation between displacement and velocity.
Let us try to obtain these equations by the graphical method.
Suppose an object is in motion along a straight line with initial velocity ‘u’. It attains
a final velocity ‘v’ in time ‘t’ due to acceleration ‘a’ its desplacement is ‘s’. The three
equations of motion can be written as
1
2
The initial velocity of the object = u = OD
The final velocity of the object = v = OC
Time = t = OE
Change in velocity
Time
Acceleration (a) =
(Final velocity – Initial velocity)
Time=
CD = at …… (i) (OC - OD = CD)
From the graph BE = AB + AE
v = CD + OD .....(AB = CD and AE = OD)
v = at + u (from i )
v = u + at
This is the first equation of motion.
(OC - OD)
t
=
Velocity
v
u
1.9 Velocity - time graph
Time
C
D
A
t
EO
B
Draw a line parallel to Y axis passing through
B. This will cross the X axis in E. Draw a line
parallel to X-axis passing through D. This will
cross the line BE at A.](https://image.slidesharecdn.com/science-and-technology-for-class-9-maharashtra-board-190829092544/85/Science-and-technology-for-class-9-maharashtra-board-9-320.jpg)













































![62
Basicity of acids : The number of H+
ions obtainable by the dissociation of one molecule
of an acid is called its basicity.
Acidity of bases : The numbers of OH-
ions obtainable by the dissociation of one molecule
of a base is called its acidity.
1. On the basis of the above table give examples of
monobasic, dibasic and tribasic acids.
2. On the basis of the above table give the three types of
bases their examples.
Concentration of acid and base
Cut a lemon into two equal parts. Take the juice
of each part into two separate beakers. Pour 10ml of
drinking water in one beaker and 20ml in the second.
Stir the solutions in both the beakers and taste them.
Is there any difference in the tastes of the solutions
in the two beakers? What is that difference?
In the above activity, the sour taste of the
solutions is because of the solute, lemon juice, in
them. The quantity of the lemon juice is the same in
both the solutions. Yet their taste is different. The
solution in the first beaker is more sour than the one
in the second. Why is it so?
Although the quantity of the solute is the same in both the solutions, the quantity of
the solvent is different. Ratio of the quantity of the solute to the quantity of the resulting
solution is different. This ratio is larger for the soultion in the first beaker and, therefore,
that solution tastes more sour. On the other hand, the proportion of the lemon juice in the
total solution in the second beaker is smaller and the taste is less sour.
The taste of foodstuff depends upon the nature of the taste-giving ingredient and its
proportion in the foodstuff. Similarly, all the properties of a solution depend on the nature
of the solute and solvent and also on the proportion of the solute in the solution. The
Proportion of a solute in a solution is called the concentration of the solute in the solution.
When the concentration of a solute in its solution is high, it is a concentrated solution,
while the solution is called a dilute solution when the concentration of the solute is low.
Several units are used to express the concentration of a solution. Two of these units
are used more frequently. The first unit is the mass of solute in grams dissolved in one litre
of the solution. (grams per litre, g/L). The second unit is the number of moles of the solute
dissolved in one litre of the solution. This is also called the molarity (M) of the solution.
The molarity of a solute is indicated by writing its molecular formula inside a square
bracket. For example [NaCl] = 1 means the molarity of this solution of common salt is 1M
(1 Molar).
5.3 Solution of lemon juice
Try this
Use your brain power !
B A](https://image.slidesharecdn.com/science-and-technology-for-class-9-maharashtra-board-190829092544/85/Science-and-technology-for-class-9-maharashtra-board-63-320.jpg)
![64
The concentration of H+
ions formed by dissociation of water at 250
C is 1 x
10-7
mol/L. At the same temperature, the concentration of H+
ions in 1M solution
of HCl is 1 x 100
mol/L. While in a 1M NaOH solution, the concentration of H+
ions is 1 x 10-14
mol/L. Thus, we see that in common aqueous solutions, the range
of H+
ion concentration is very wide from 100
to 10-14
mol/L. In 1909, the Danish
scientist Sorensen introduced a convenient new scale of expressing H+
ion
concentration which is found to be very useful in chemical and biochemical
processes. It is the pH scale (pH : power of hydrogen) The pH scale extends from
0 to 14. According to this scale pure water has a pH of 7 meaning pure water
has [H+
] = 1 x 10-7
mol/L. pH 7 indicates a neutral solution. This pH is the midpoint
of the scale. The pH of an acidic solution is less than 7 and that of a basic solution
is greater than 7. The table (See page no. 63) gives the pH values of some
common solutions.
By which other method could we find out the pH of a solution?
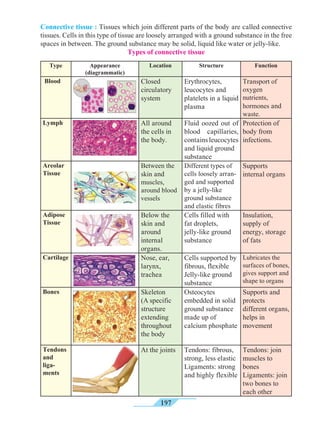
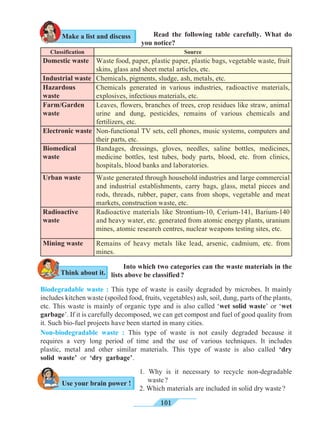
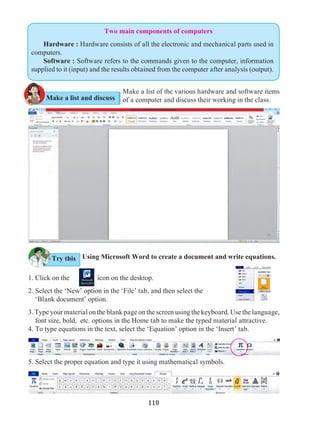
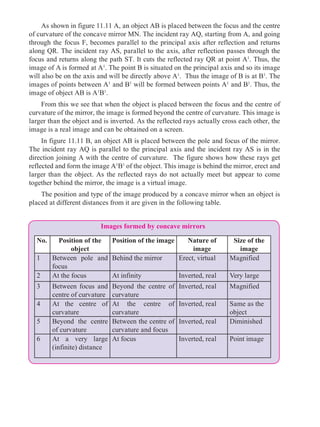
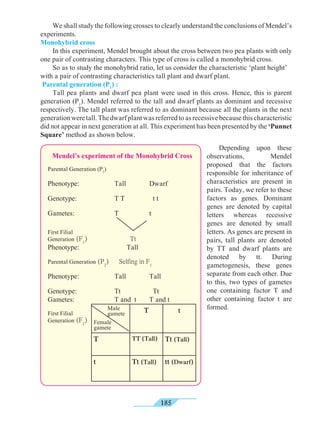
Universal indicators
5.4 Measurement of pH : universal indicator and pH meter
What are the colours of the following natural and
synthetic indicators in acidic and basic solutions ?
Litmus, turmeric, jamun, methyl orange, phenolphthalein ?
A universal indicator is made by mixing several synthetic indicators in specific
proportions. The pH of a solution can be determined by means of a universal
indicator solution or the pH paper made from it. However, the most accurate method
of measuring the pH of a solution is to use an electrical instrument called pH
meter. In this method, pH is measured by dipping electrodes into the solution.
BasicAcid
Neutral
Can you recall?
We know that some natural and synthetic dyes show two different colours in acidic
and basic solutions, and such dyes are used as acid-base indicators. In the pH scale, the
pH of solutions varies from 0 to 14 in accordance with the strength of the acid or base. To
show these variations in pH, a universal indicator is used. A universal indicator shows
different colours at different values of pH.](https://image.slidesharecdn.com/science-and-technology-for-class-9-maharashtra-board-190829092544/85/Science-and-technology-for-class-9-maharashtra-board-65-320.jpg)



































































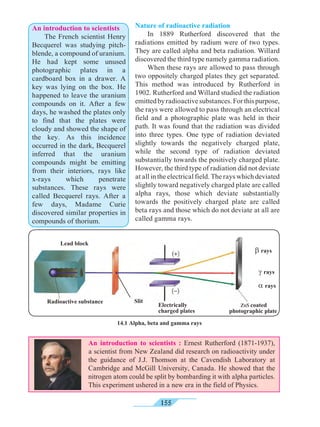













![192
C. Mitochondrial disorder :
Mitochondrial DNA may also become defective due to mutation. During fertilization,
mitochondria are contributed by the egg cell (ovum) alone. Hence, mitochondrial disorders
are inherited from the mother only. Leber hereditary optic neuropathy is an example of a
mitochondrial disorder.
D] Disorders due to mutations in multiple genes : (Polygenic disorders)
Sometimes, disorders arise due to mutations in more than one gene. In most such
disorders, their severity increases due to effects of environmental factors on the foetus.
Common examples of such disorders are cleft lip, cleft palate, constricted stomach, spina
bifida (a defect of the spinal cord), etc. Besides, diabetes, blood pressure, heart disorders,
asthma, obesity are also polygenic disorders. Polygenic disorders do not strictly follow
Mendel’s principles of heredity. These disorders arise from a complex interaction between
environment, life style and defects in several genes.
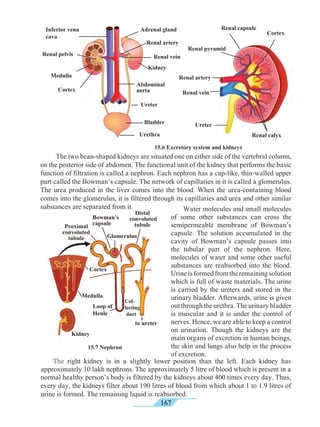
Compose and present a
street play against tobacco
consumption and participate
in a drive against tobacco.
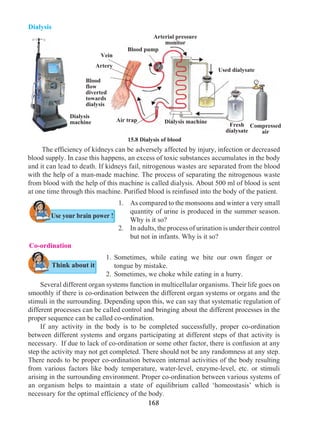
Always remember
Inter-relationship between tobacco addiction and
cancer (Uncontrolled growth of cells)
Many people consume tobacco, either by smoking or by
chewing. Consumption of tobacco in any form can cause
cancer. Smoking of cigarettes and bidi adversely affects the
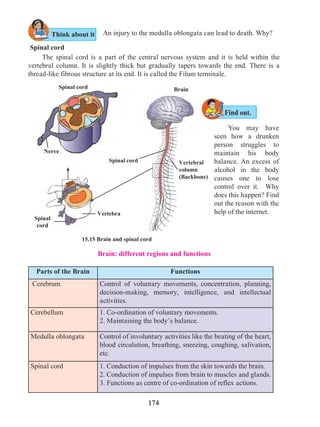
process of digestion. It causes a burning sensation in the
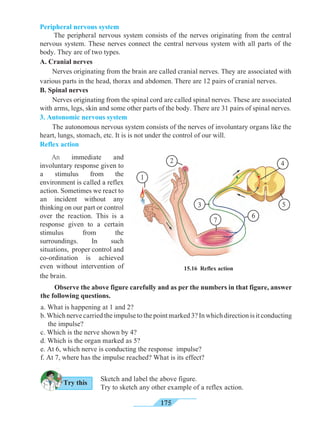
throat and a cough. Excessive smoking causes instability
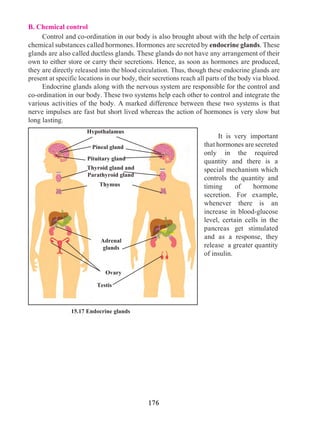
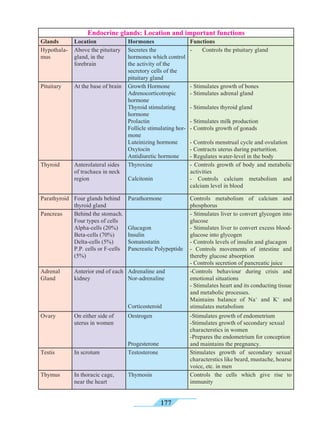
and trembling of fingers. A dry cough causes sleeplessness.
Tobacco consumption can also lead to shortening of life
span, chronic bronchitis, pericarditis, cancer of the lungs,
mouth, larynx (voice box), pharynx, pancreas, urinary
bladder, etc.
Harmful effects of smoking are due to the nicotine
present in tobacco. It affects the central and peripheral
nervous system. Arteries become hard i.e. it causes
arteriosclerosis and hypertension.
Tobacco smoke contains harmful chemicals like
pyridine, ammonia, aldehyde furfural, carbon monoxide,
nicotine, sulphur dioxide, etc. They cause uncontrolled cell
division. Tobacco smoke is full of minute carbon particles
which causes normal tissue of the lung to transform into
thickened black tissue. This causes cancer. While chewing
tobacco or tobacco products much of the extract is absorbed
into the body. Excessive tobacco consumption may cause
cancer of lips or tongue, visual disorders or tremors.
To protect one’s body from cancer one must avoid smoking
and consumption of tobacco and tobacco products in any
form.](https://image.slidesharecdn.com/science-and-technology-for-class-9-maharashtra-board-190829092544/85/Science-and-technology-for-class-9-maharashtra-board-193-320.jpg)