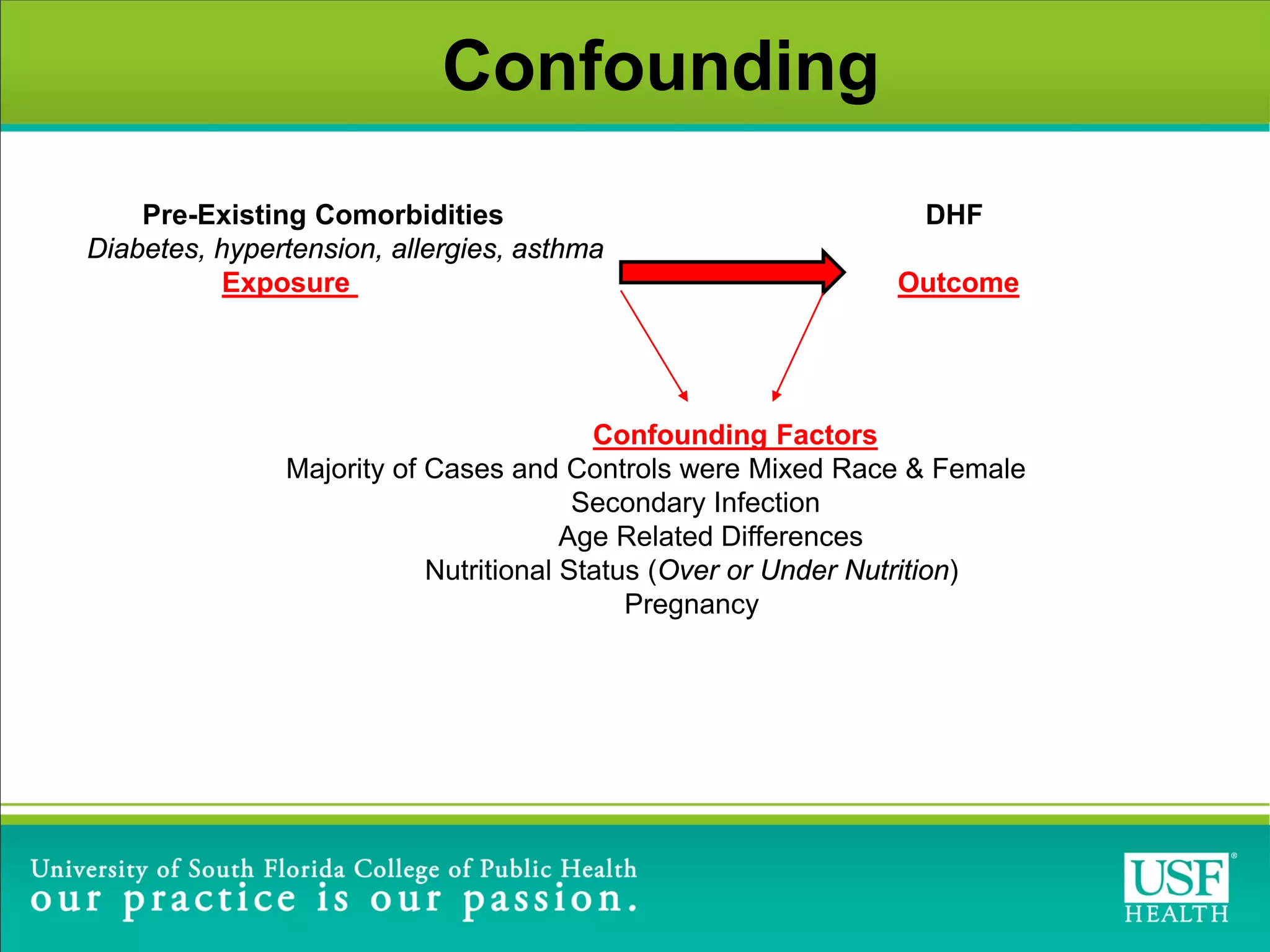
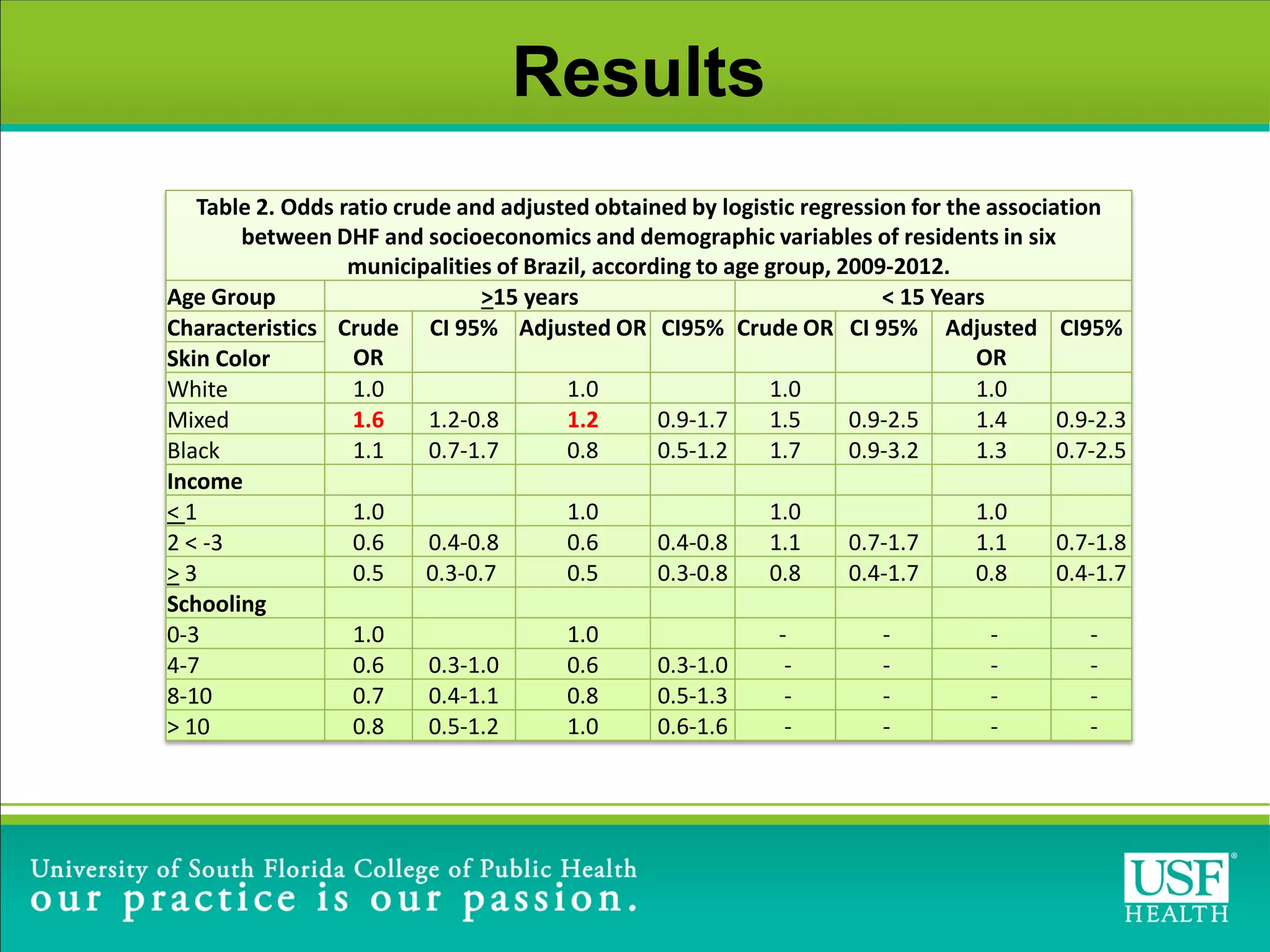
This study investigated risk factors for progression from dengue fever (DF) to dengue hemorrhagic fever (DHF) in Brazil between 2009-2012. The study found that arterial hypertension and skin allergies were significant risk factors for developing DHF after being diagnosed with DF, with adjusted odds ratios of 1.6 and 1.8 respectively. The study highlights the need for close monitoring of DF patients with these comorbidities during dengue outbreaks to help identify potential DHF cases early and provide appropriate clinical management.