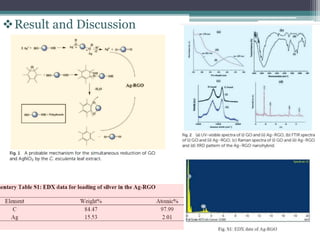
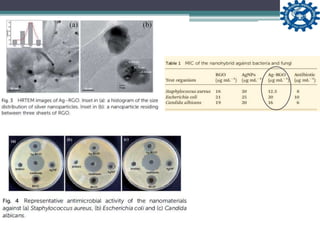
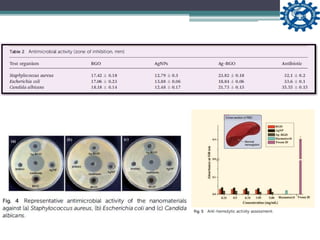
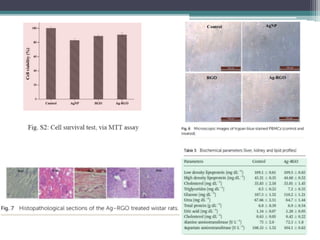
This document describes the preparation and characterization of a biocompatible, antimicrobial reduced graphene oxide-silver nanohybrid (Ag-RGO) using a green method involving colocasia esculenta leaf extract. The study evaluates its antimicrobial efficacy, cytotoxicity, and acute dermal toxicity in Wistar rats, confirming its potential as a topical antimicrobial agent for biomedical applications. The findings indicate the nanohybrid's compatibility with mammalian cells and its promise for use in medical products such as bandages and ointments.