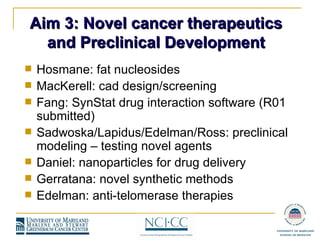
The document summarizes the activities of the Experimental Therapeutics program at an annual retreat. It discusses the program's aims of identifying new drug targets and mechanisms of drug resistance. It provides an overview of clinical trials and preclinical research underway, including studies on cell cycle targets, drug sensitivity and resistance, and novel cancer therapeutics. The document also reviews funding, publications, and plans to renew the program's P30 grant.













![P30 Critique – Summary Broad programmatic effort without adequate focus on: Target Methodological approach Preclinical model assessment Drug discovery in clinical trials Need for increased investigator-initiated clinical trials that derive from the laboratories of the Program “… Program leaders [have not] guided the membership towards coordinated approaches that will lead to multi-investigator scientific initiatives and grants” “ It is not convincing that the Program will make significant progress in the near future or add considerably to the national effort in discovery and development of important anti-cancer drugs.”](https://image.slidesharecdn.com/ross-etoverview-retreat2009-091005105542-phpapp02/85/Ross-ET-Overview-17-320.jpg)