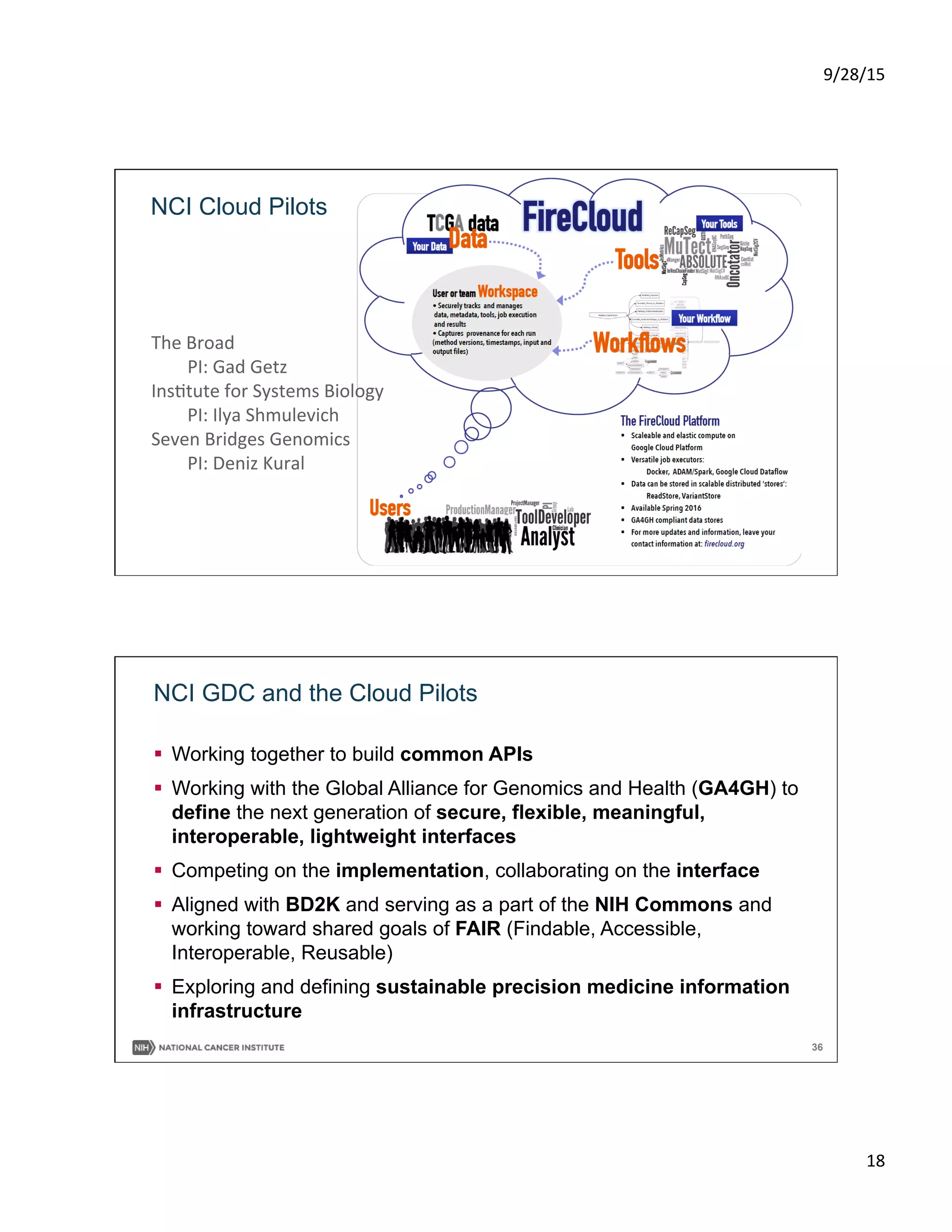
This document summarizes a presentation on precision medicine in oncology from an informatics perspective. It discusses the goals of precision oncology to target cancer treatments based on a patient's individual tumor characteristics. Major initiatives are described, including the NCI-MATCH trial which assigns cancer therapies based on a tumor's molecular abnormalities. The presentation outlines efforts through the Precision Medicine Initiative to expand genomic cancer trials, understand and overcome resistance to therapies through additional tumor profiling and preclinical models, and establish an integrated national cancer database.






![9/28/15
9
17
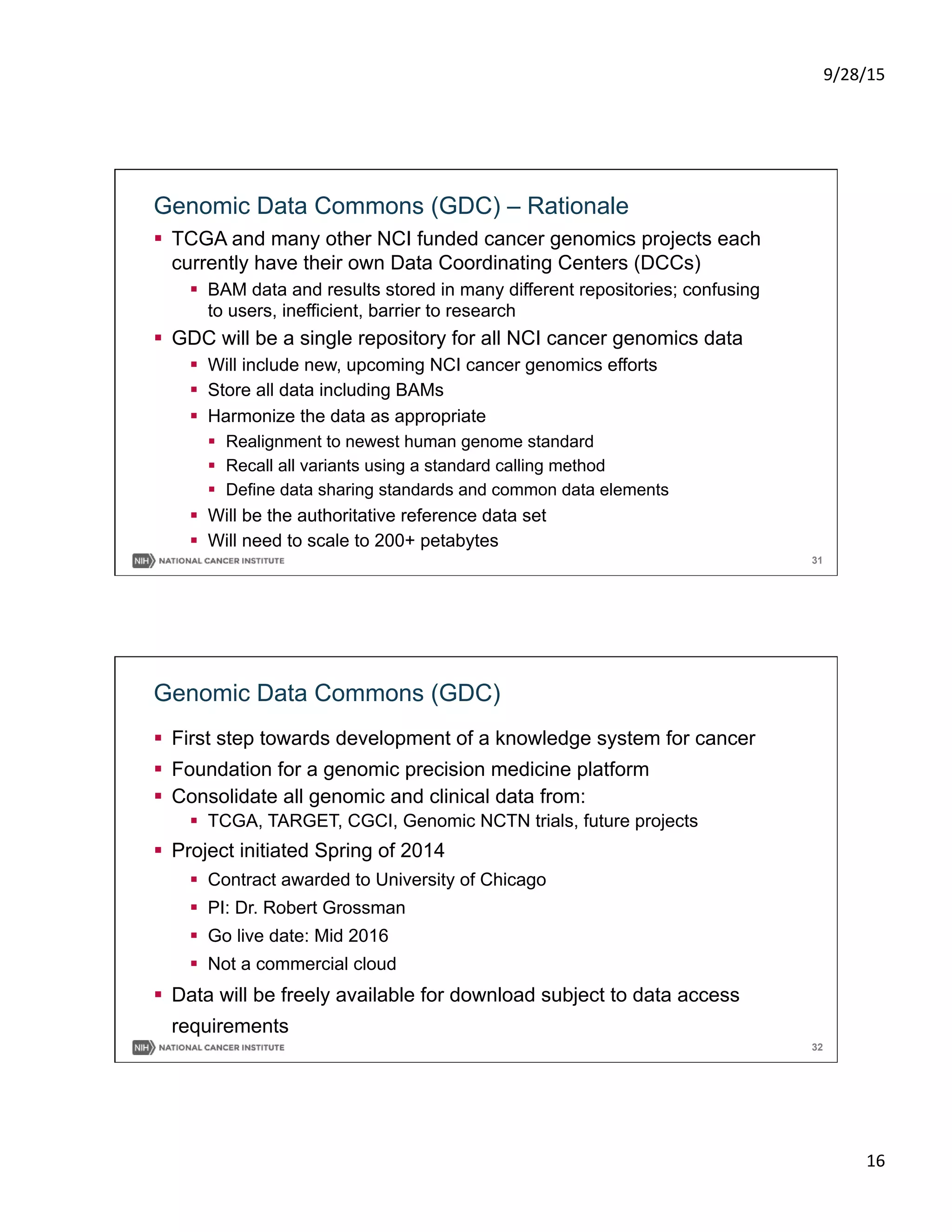
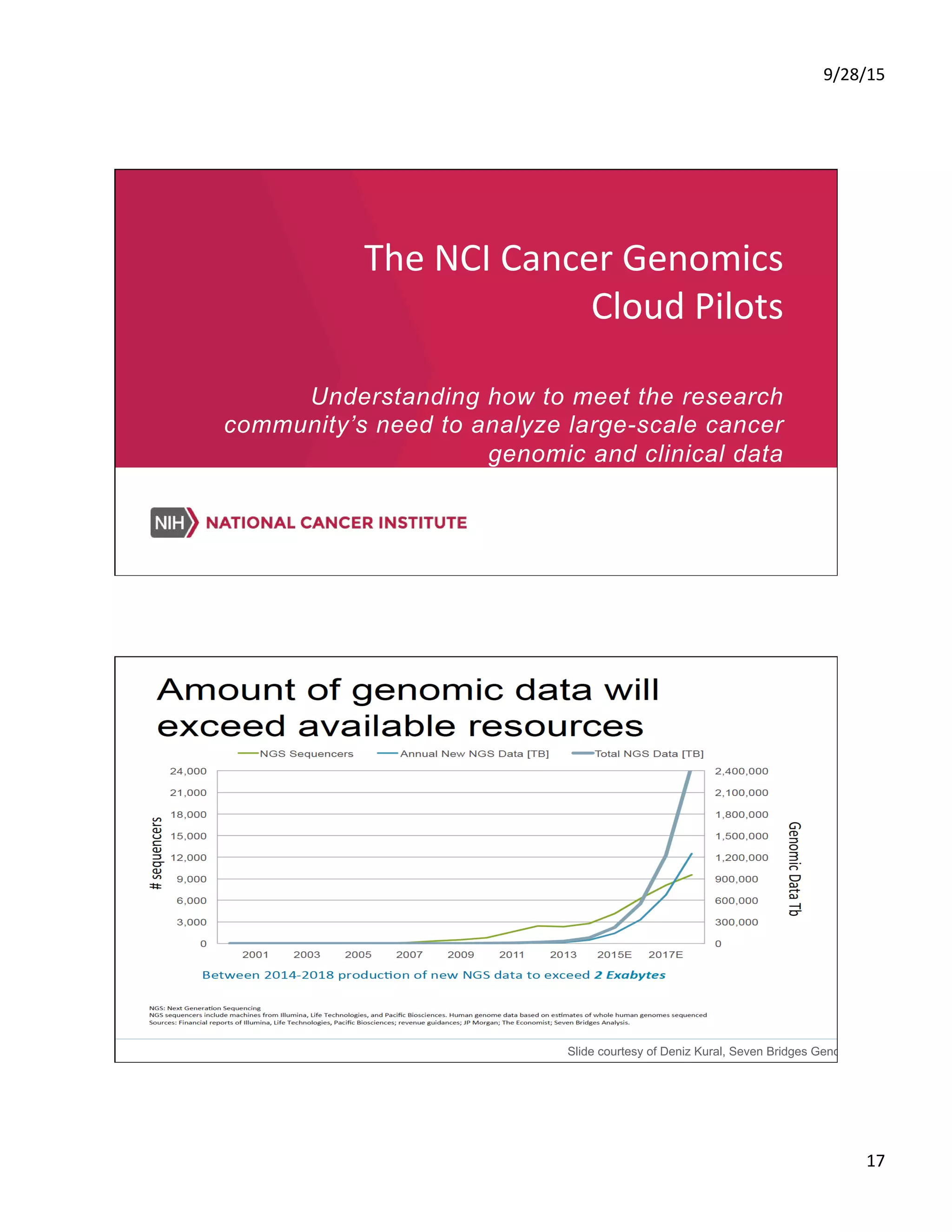
Precision Oncology
Trials Launched
2014:
MPACT
Lung MAP
ALCHEMIST
Exceptional Responders
2015:
NCI-MATCH
ALK Inhibitor
MET Inhibitor
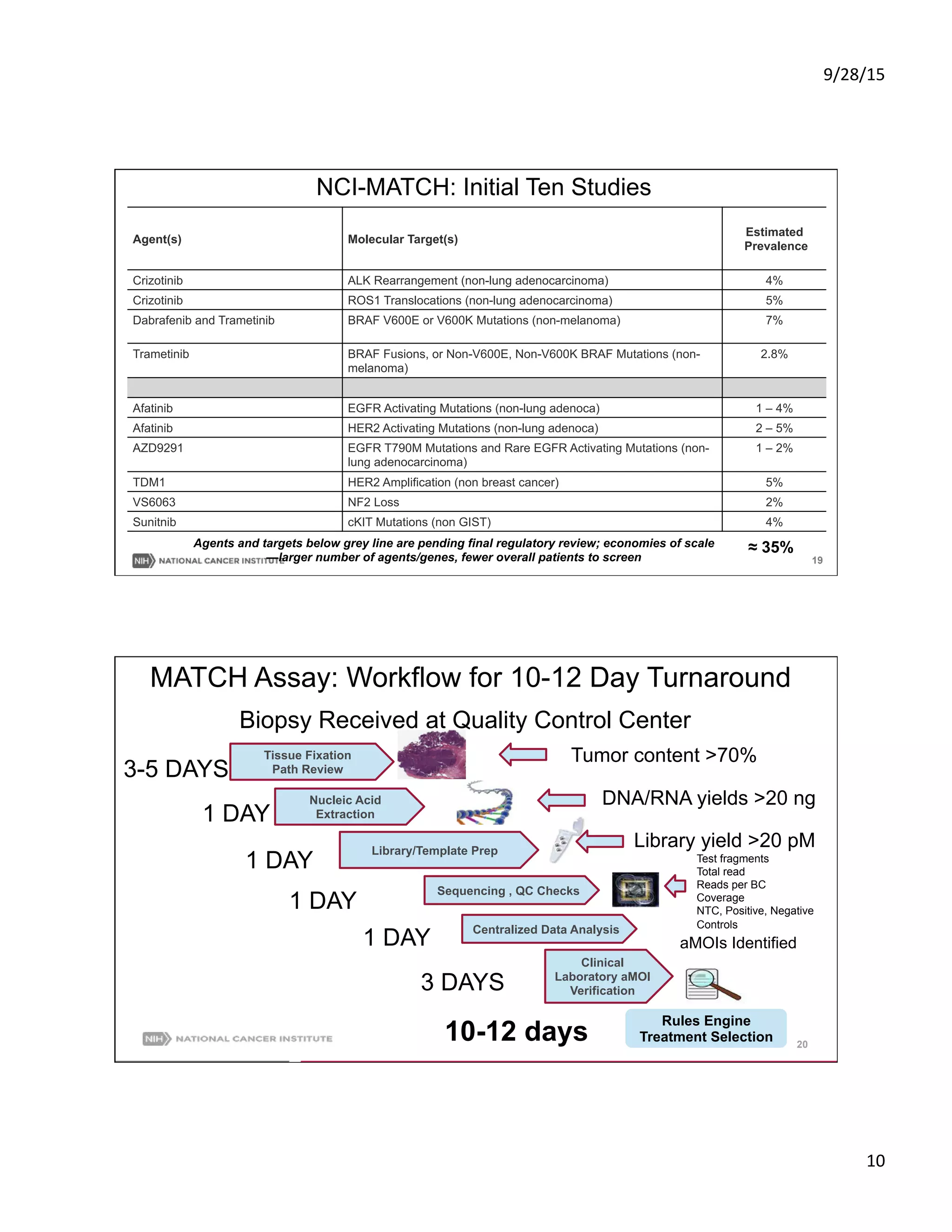
NCI-MATCH: Features
[Molecular Analysis for Therapy Choice]
• Foundational treatment/discovery
trial; assigns therapy based on
molecular abnormalities, not site of
tumor origin for patients without
available standard therapy
• Regulatory umbrella for phase II
drugs/studies from > 20 companies;
single agents or combinations
• Available nationwide (2400 sites)
• Accrual began mid-August 2015
NCI MATCH
• Conduct
across
2400
NCI-‐supported
sites
• Pay
for
on-‐study
and
at
progression
biopsies
• Ini5al
es5mate:
screen
3000
pa5ents
to
complete
20
phase
II
trials
1CR,
PR,
SD,
and
PD
as
defined
by
RECIST
2Stable
disease
is
assessed
relaSve
to
tumor
status
at
re-‐iniSaSon
of
study
agent
3Rebiopsy;
if
addiSonal
mutaSons,
offer
new
targeted
therapy
,2](https://image.slidesharecdn.com/pmi-okibbeseptdoe-print-150928183707-lva1-app6892/75/Precision-Medicine-in-Oncology-Informatics-9-2048.jpg)