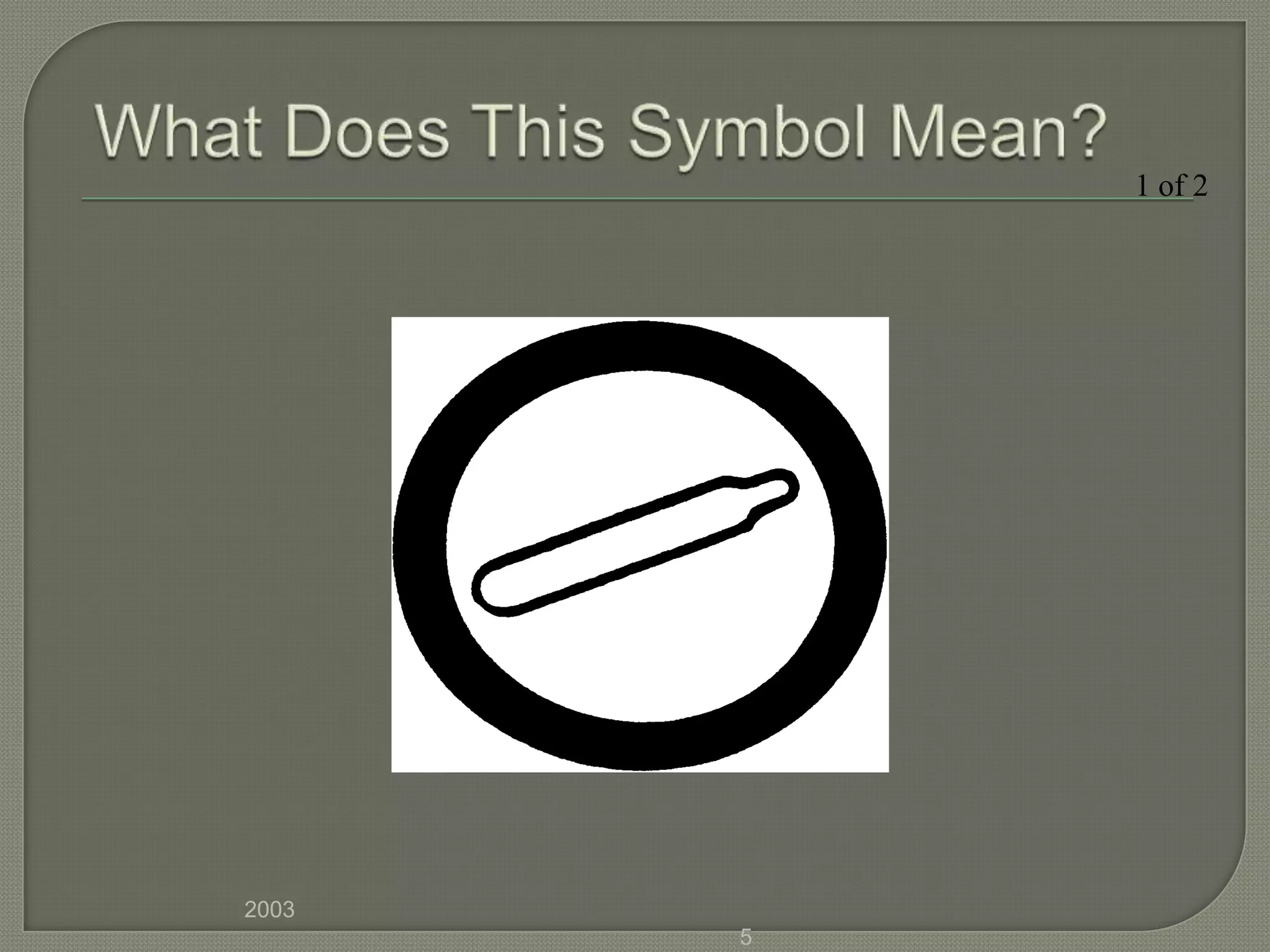
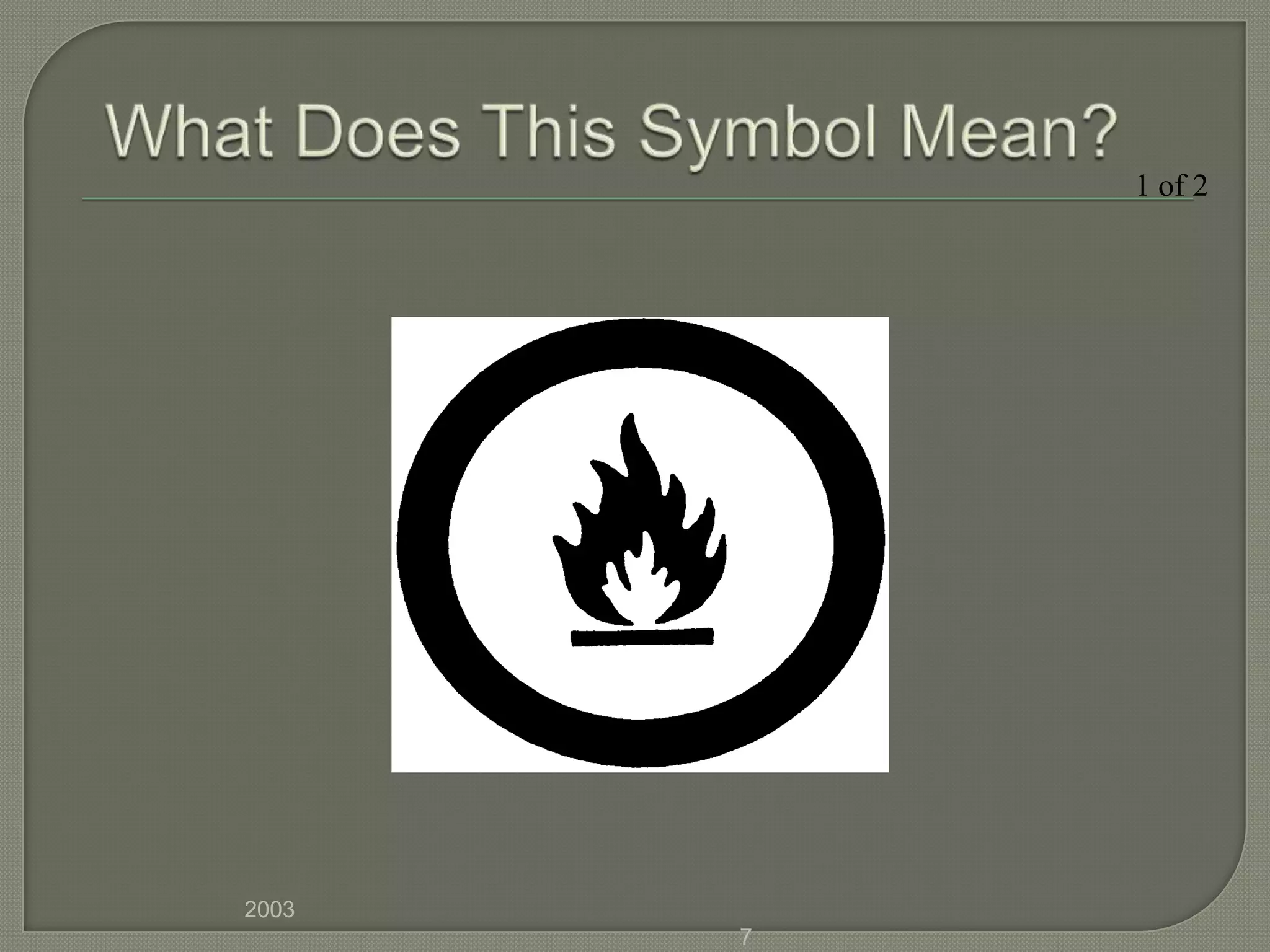
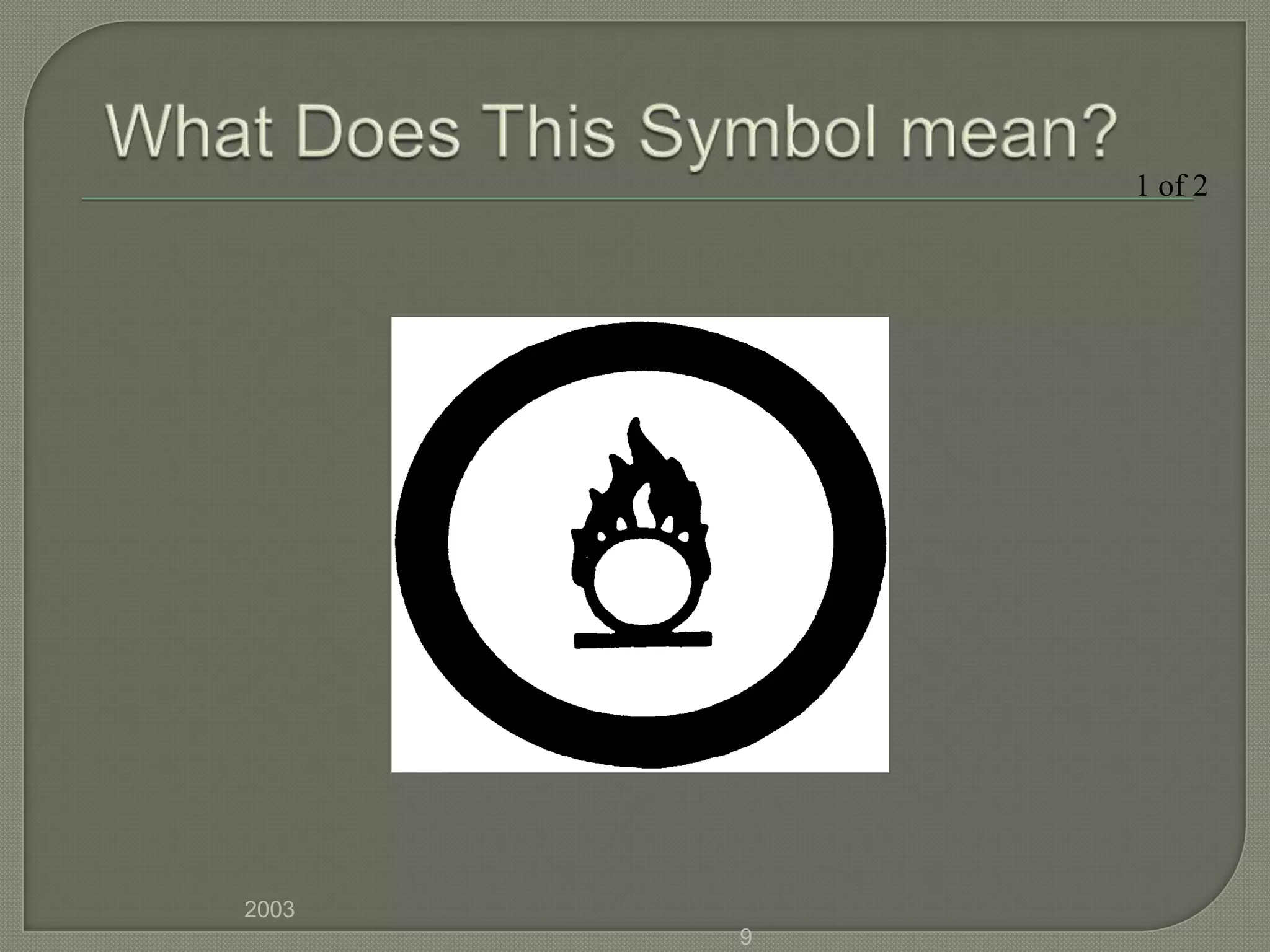
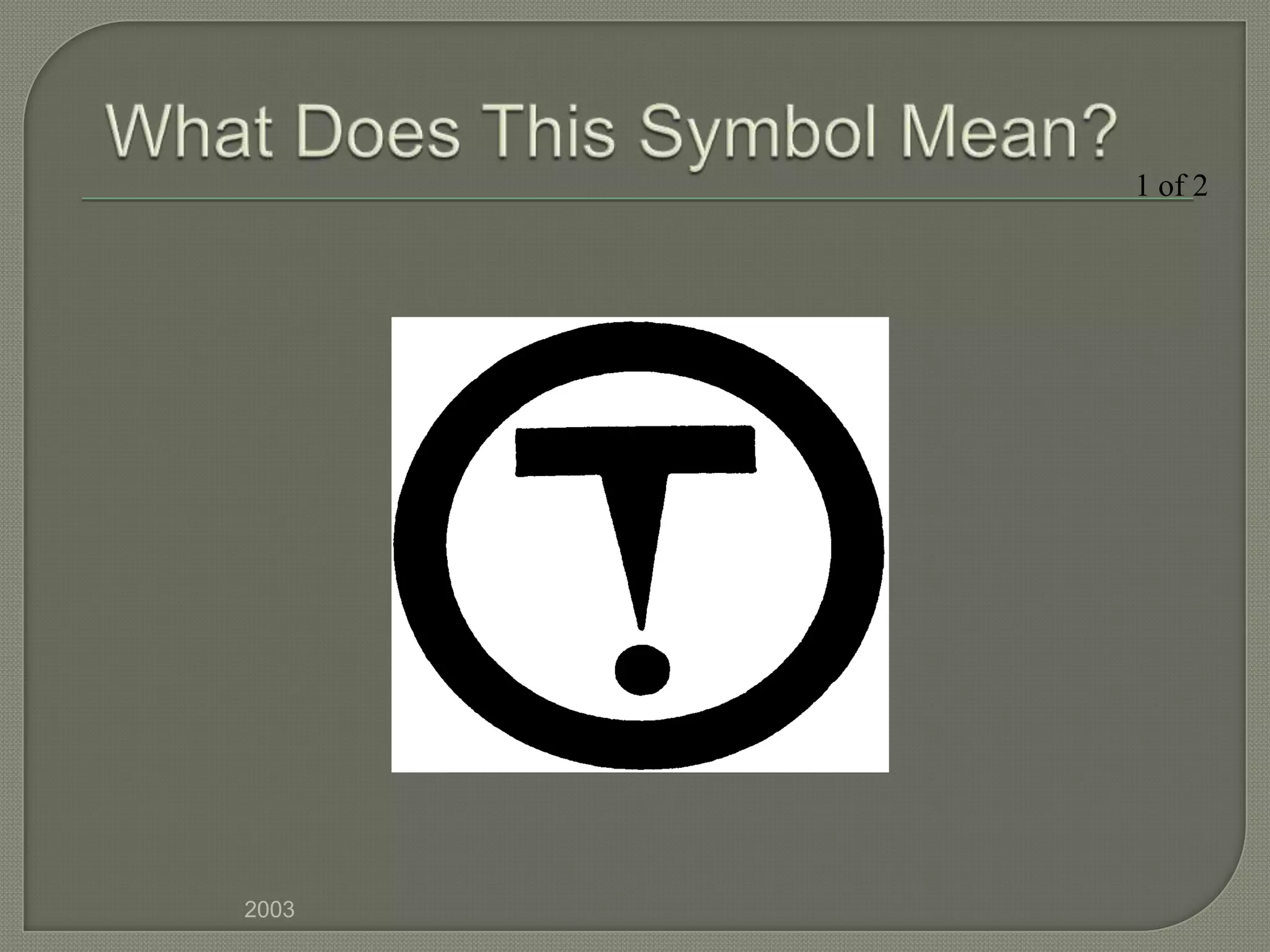
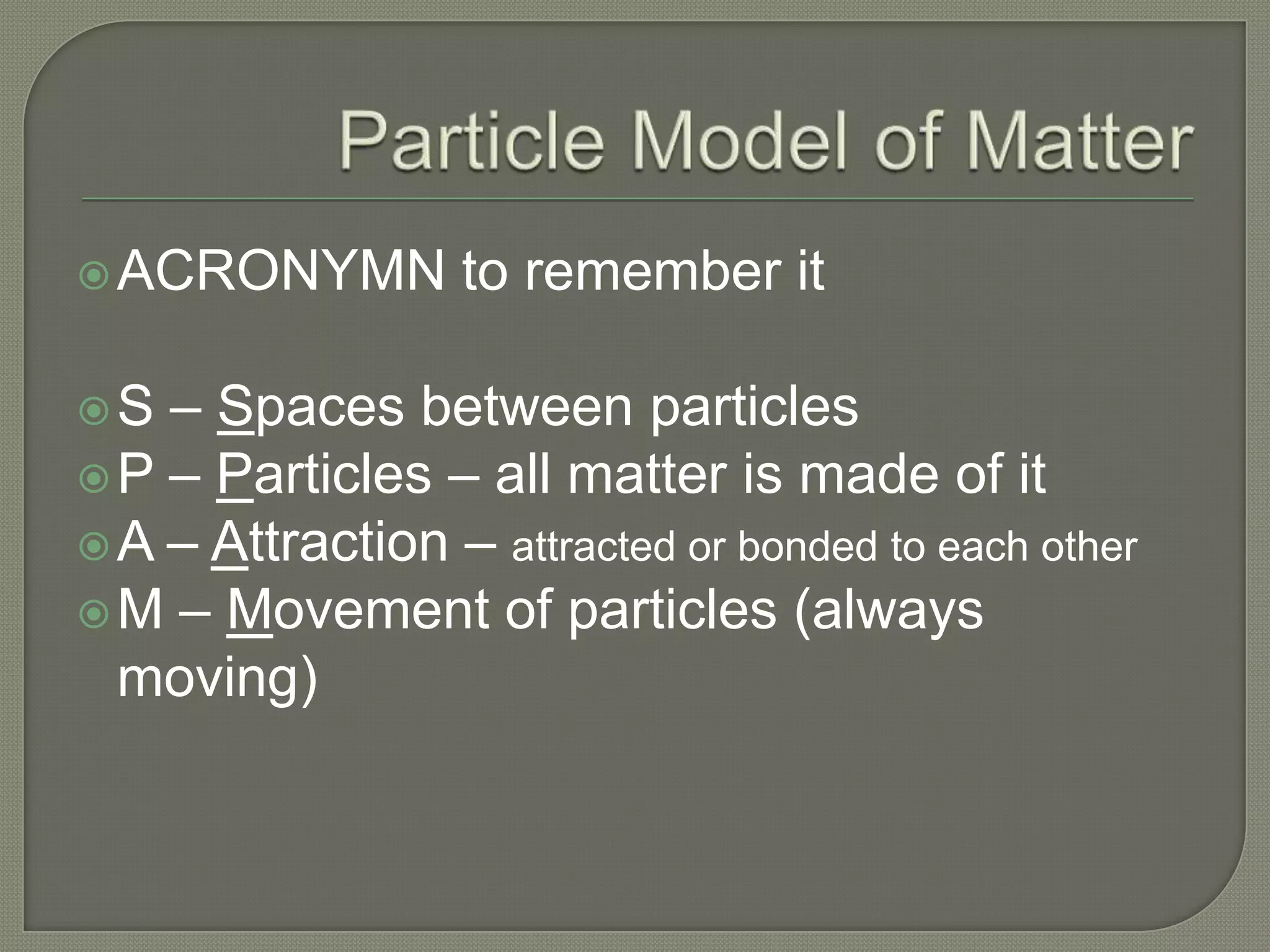
The document outlines the different hazard classes used to classify dangerous goods, including compressed gases, flammable and combustible materials, oxidizing materials, poisonous and infectious materials, corrosive materials, and dangerously reactive materials. Each hazard class is defined and examples of the types of materials that fall into each class are provided. Common hazard symbols associated with each class are also displayed and explained.