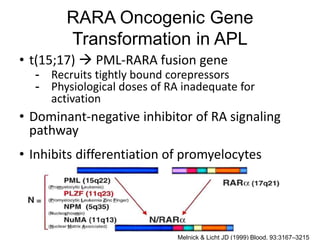
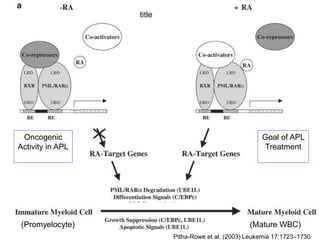
The RARA gene encodes for the retinoic acid receptor alpha, which is a transcription factor activated by retinoic acid and is critical for cellular differentiation and proliferation. In acute promyelocytic leukemia (APL), a translocation mutation creates a dominant-negative fusion protein (PML-RARA) that inhibits normal differentiation of promyelocytes, leading to leukemia. Treatments like all-trans retinoic acid (ATRA) can restore differentiation by degrading the fusion protein and activating differentiation pathways.


![References
• Bastien J, Rochette-Egly C. 2004. Nuclear retinoid receptors and the transcription of retinoid-target genes
Gene. Gene 328:1–16.
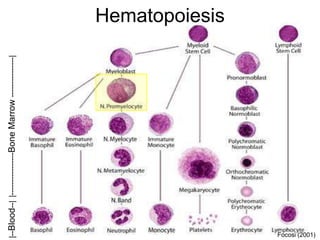
• Focosi D. 2001. Physiology of adult homo sapiens - blood (haematology : plasma, blood cells, and
coagulation) and lymph [Internet]. Available from:
http://www.ufrgs.br/imunovet/molecular_immunology/blood.html
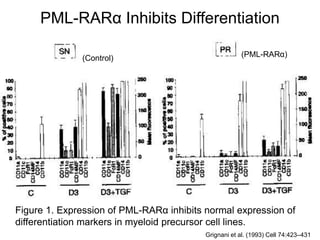
• Grignani Francesco, Ferucci PF, Testa U, Talamo G, Fagioli M, Alcalay M, Mencarelli A, Grignani Fausto,
Peschle C, Nicholetti I, et al. 1993. The acute promyelocytic leukemia-specific PML-RARα fusion protein
inhibits differentiation and promotes survival of myeloid precursor cells Cell. Cell 74:423–431.
• Lingfelder E, Niederwieser D, Platzbecker U, Schlenk RF, Wörmann J. 2012. Acute Promyelocytic Leukemia
(APL) [Internet]. (onkopedia, editor.). Available from: https://www.onkopedia-
guidelines.info/en/onkopedia/guidelines/acute-promyelocytic-leukemia-
apl/@@view/html/index.html#ID0EG
• Melnick A, Licht JD. 1999. Deconstructing a Disease: RARα, Its Fusion Partners, and Their Roles in the
Pathogenesis of Acute Promyelocytic Leukemia Blood. Blood 93:3167–3215.
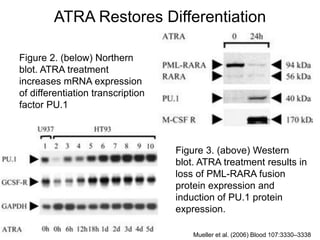
• Mueller BU, Pabst T, Fos J, Fey MF, Asou N, Buergi U, Tenen DG. 2006. ATRA resolves the differentiation
block in t(15;17) acute myeloid leukemia by restoring PU.1 expression Blood. Blood 107:3330–3338.
• Niederreither K, Dolle P. 2008. Retinoic acid in development: towards an integrated view Nature Reviews
Genetics. Nature 9:541–553.
• Pitha-Rowe I, Petty WJ, Kitaweeran S, Dmitrovsky E. 2003. Retinoid target genes in acute promyelocytic
leukemia Leukemia. Leukemia 17:1723–1730.
• Vigue F. 2010. RARA (Retinoic acid receptor, alpha) [Internet]. (Atlas of Genetics and Cytogenetics in
Oncology and Haematology, editor.). Available from:
http://atlasgeneticsoncology.org/Genes/RARAID46.html](https://image.slidesharecdn.com/5rarabeard-160421171442/85/Retinoic-Acid-Receptor-Alpha-13-320.jpg)