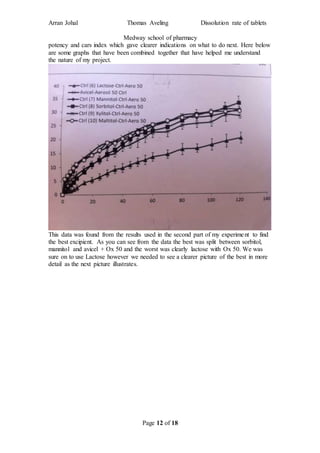
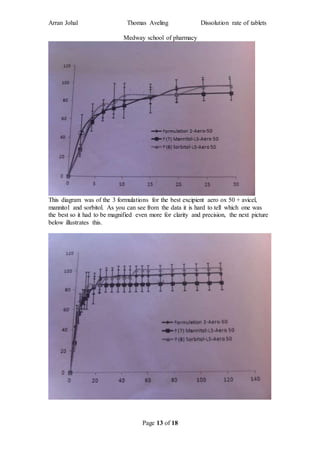
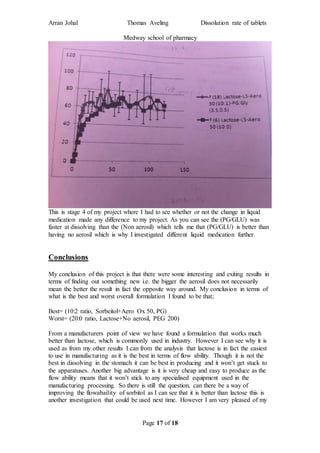
The student completed a work placement at the Medway School of Pharmacy where they investigated methods to improve the dissolution rate of poorly soluble drugs. In the first part of the project, the student tested different types of coating materials (aerosil 50, 130, 200, 300, 380) while keeping other variables constant. Testing in a dissolution apparatus showed that smaller surface area coating materials resulted in better drug release. In the second part, the student investigated changing the excipient (carrier material) while keeping other factors the same.