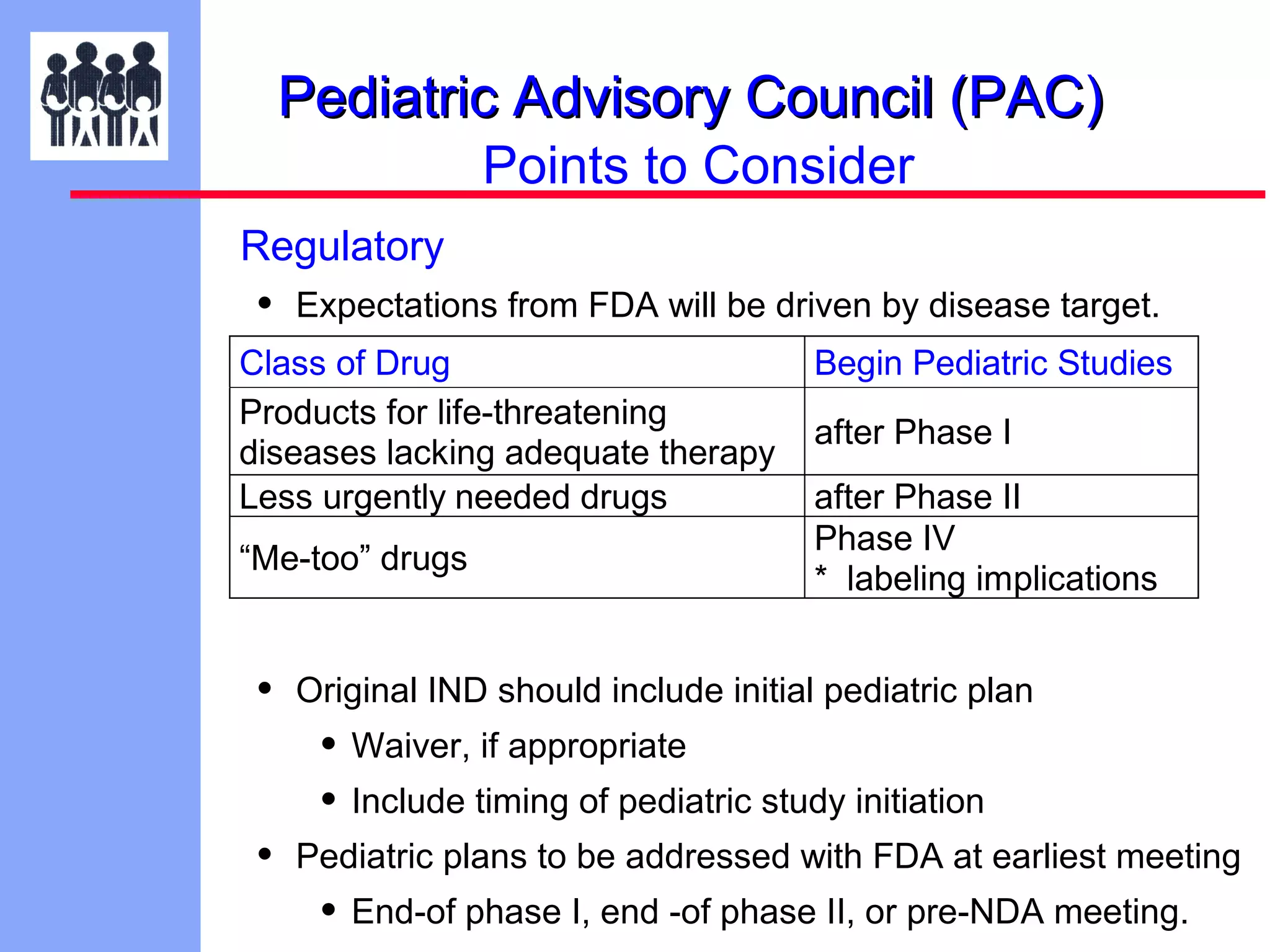
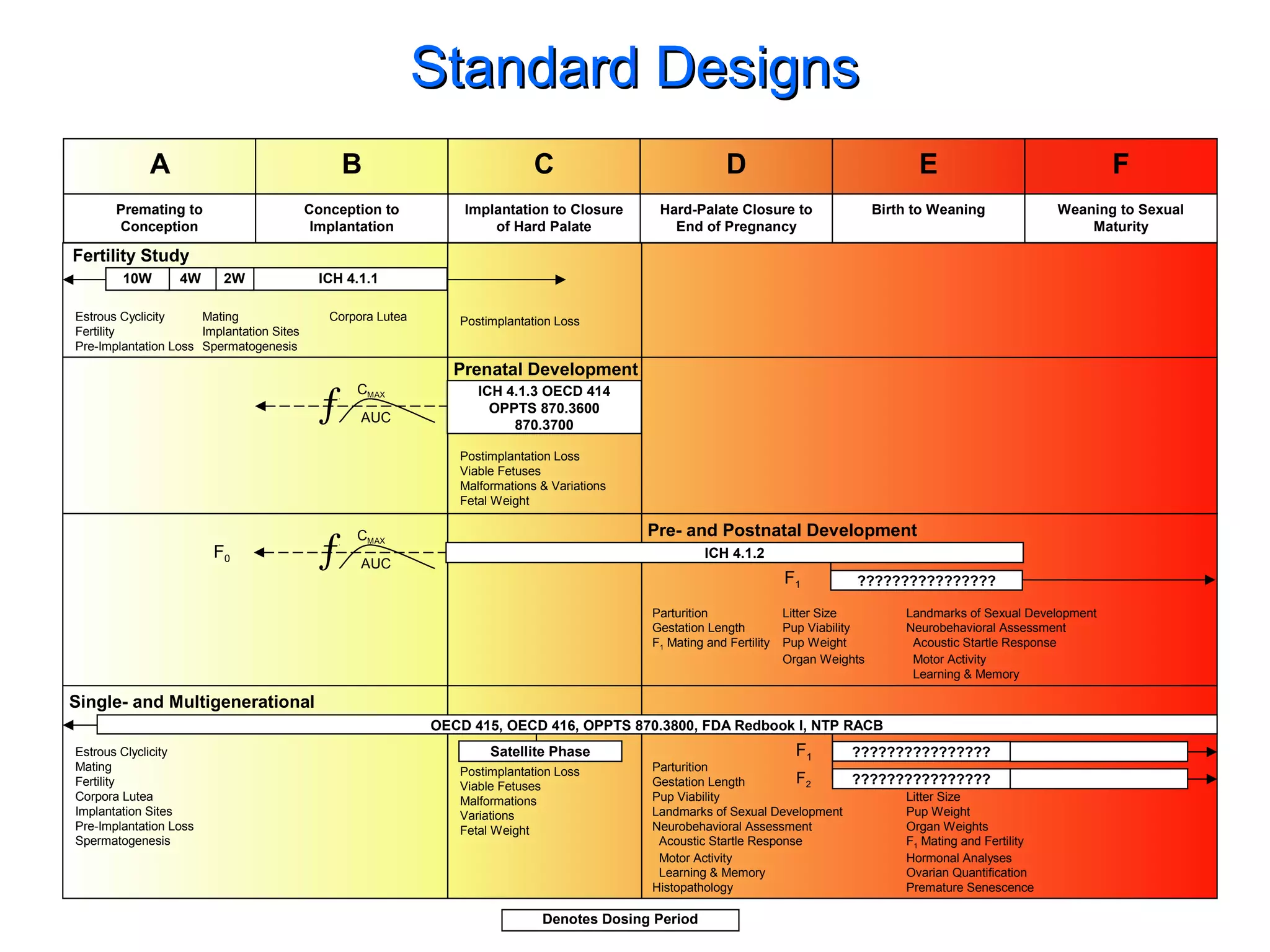
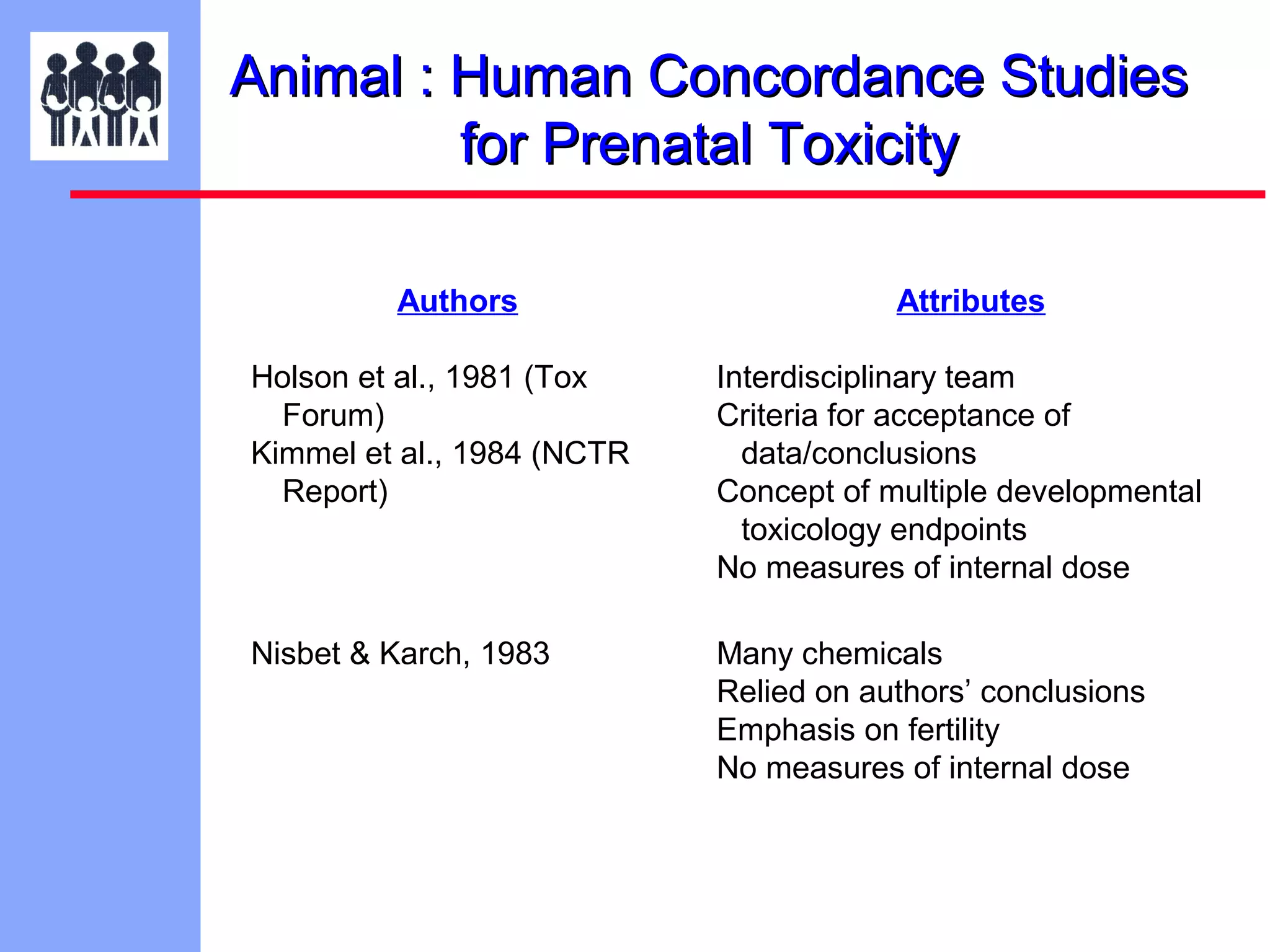
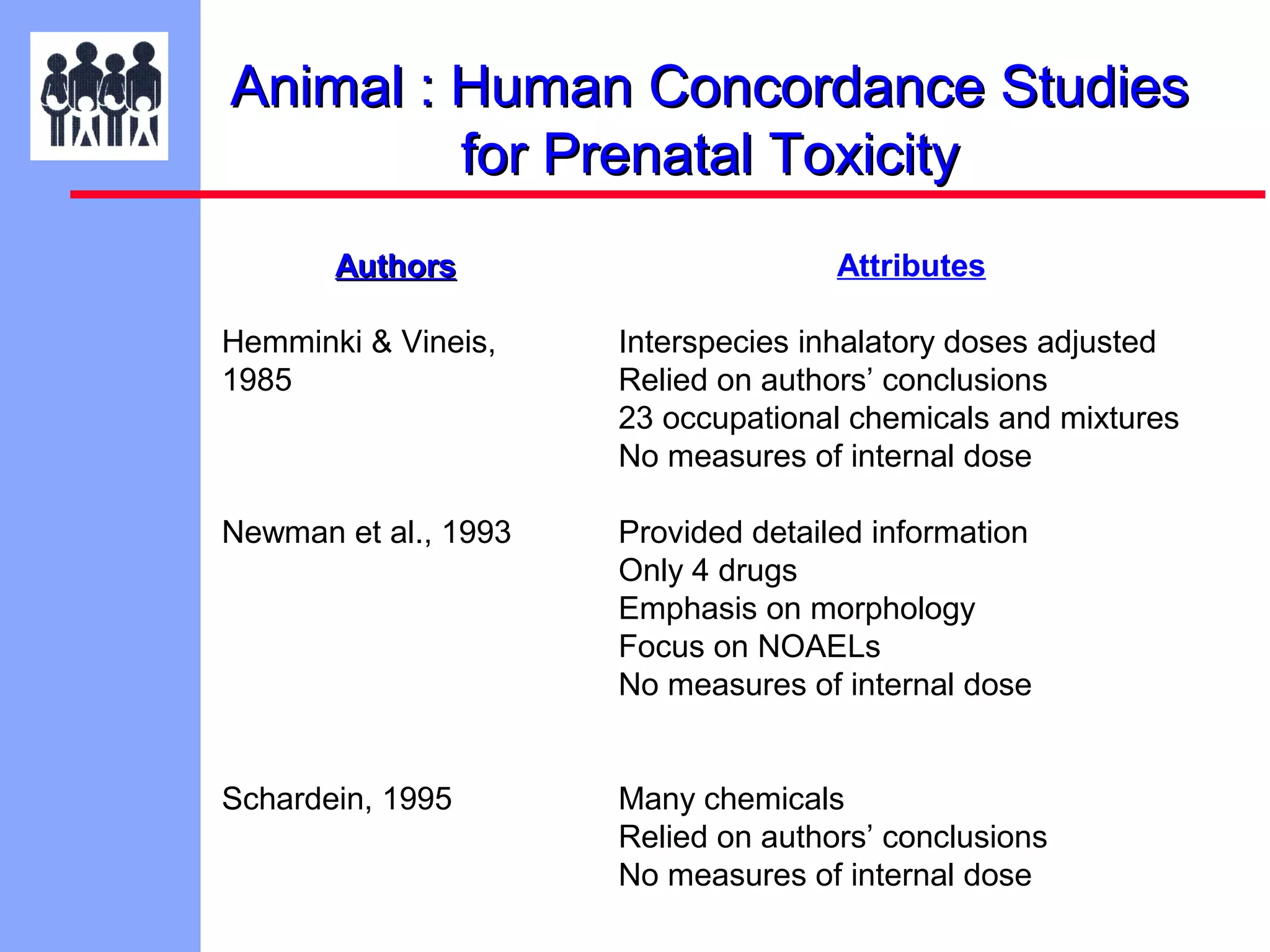
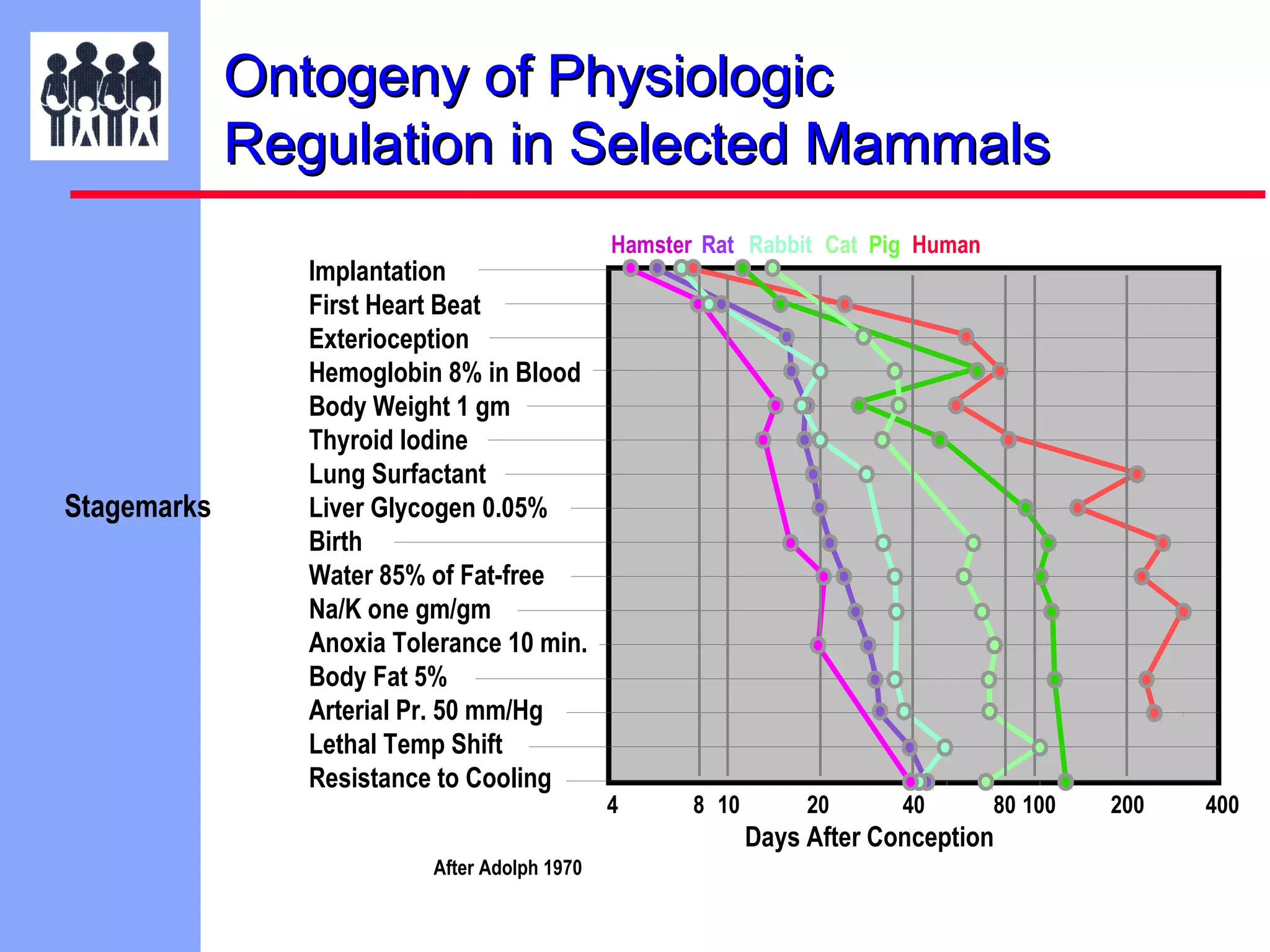
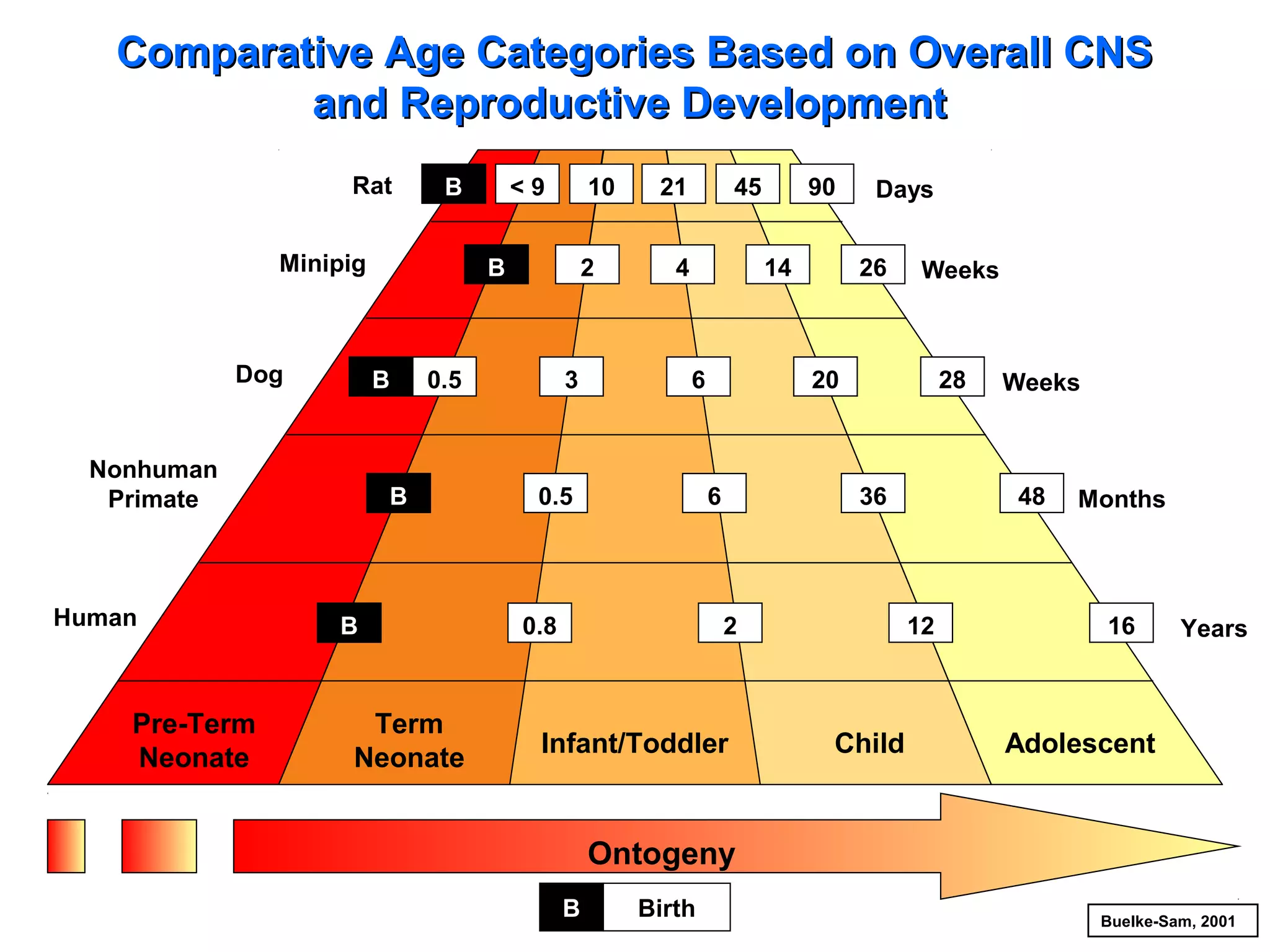
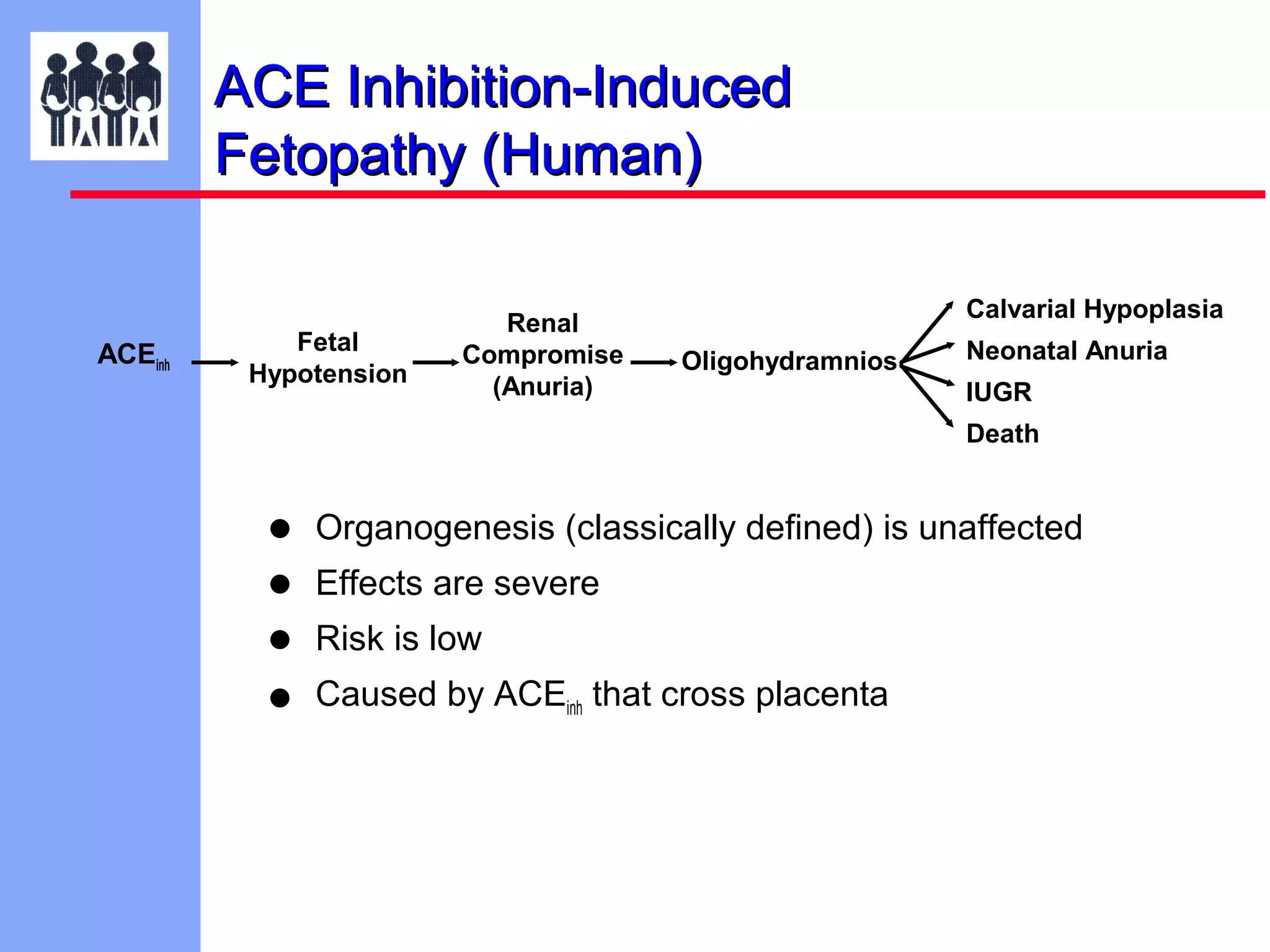
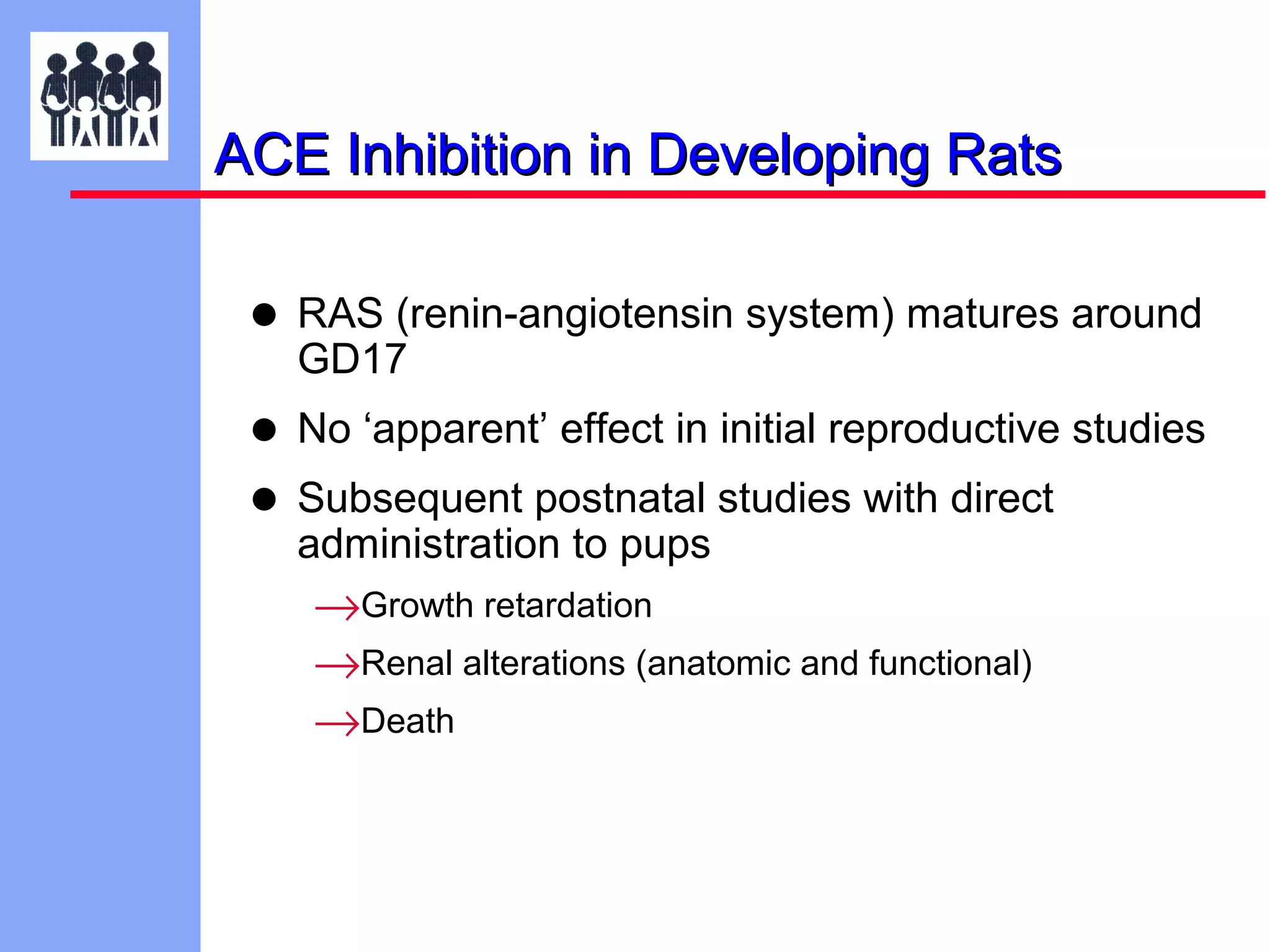
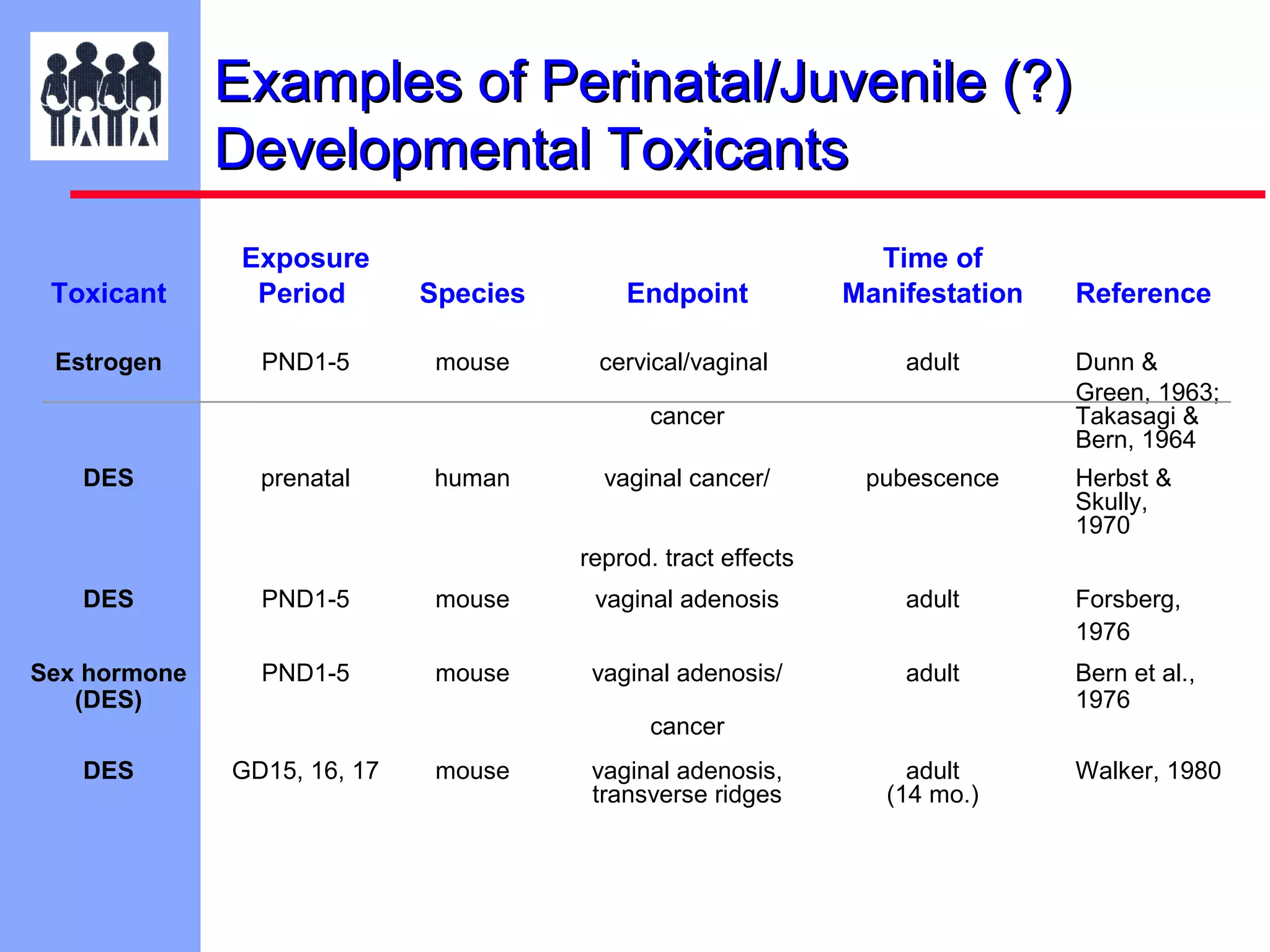
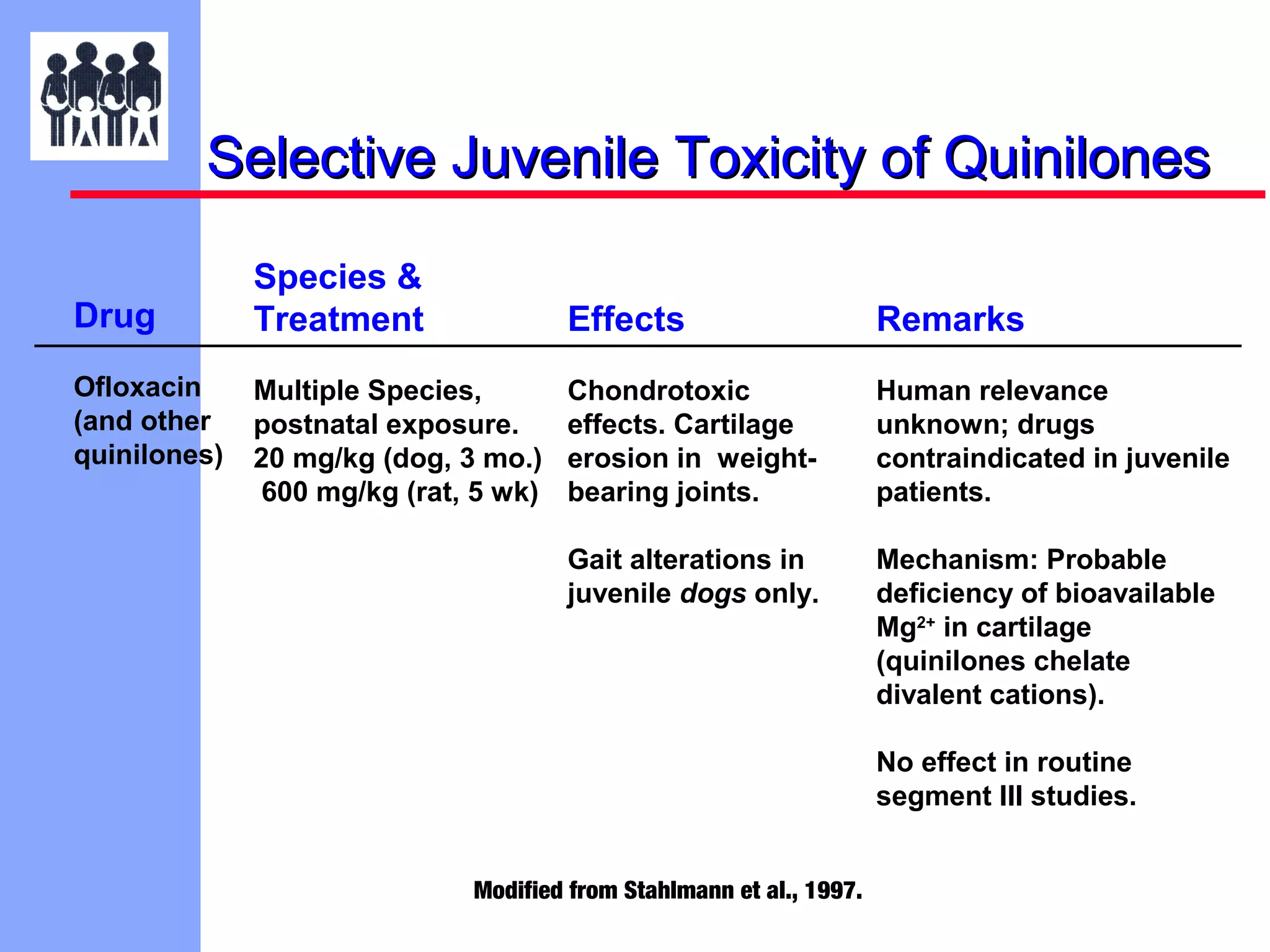
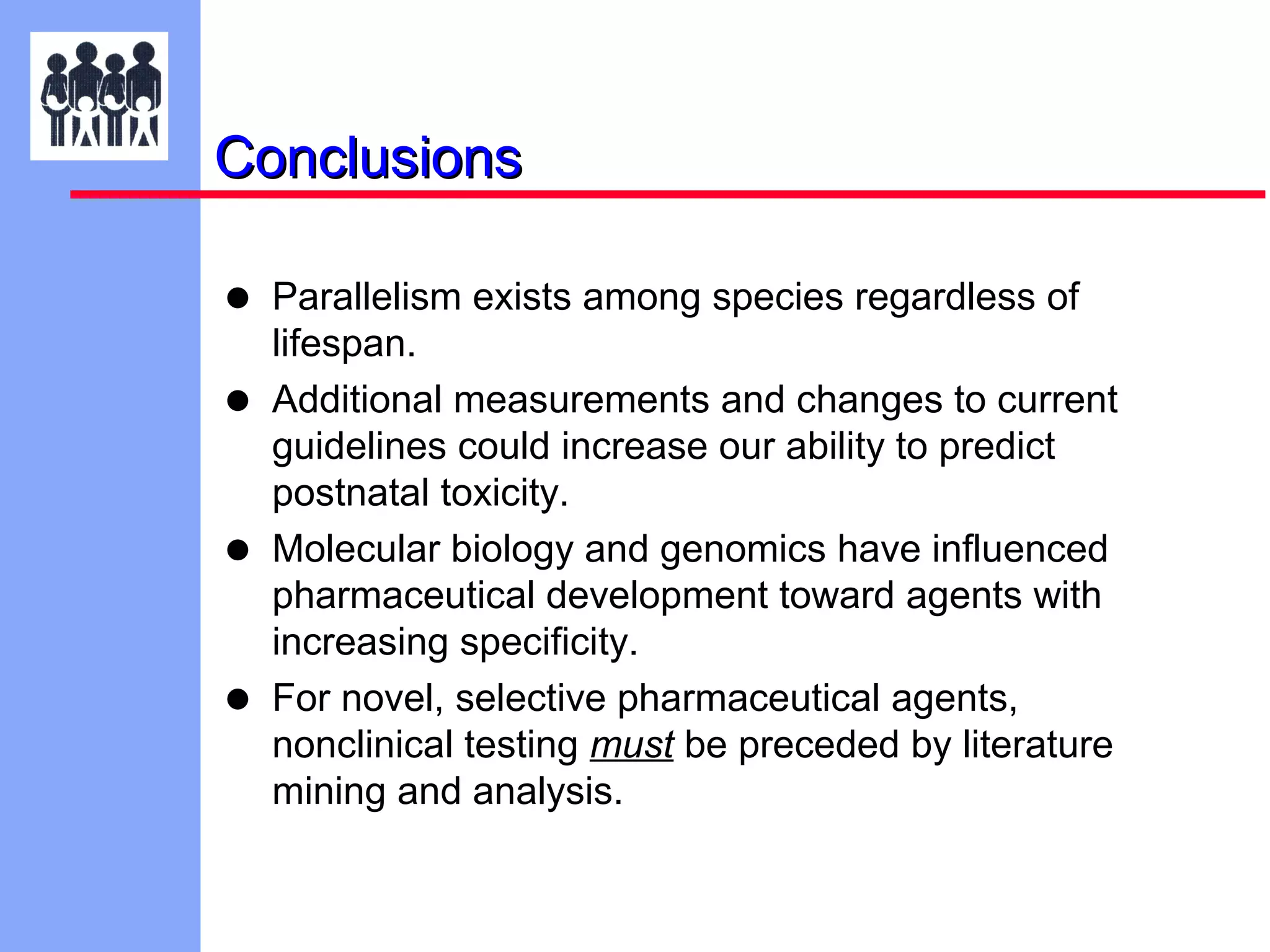
The document discusses the regulatory framework established by the FDA for assessing the safety and effectiveness of drugs and biological products in pediatric patients as mandated by the FDAMA and the Pediatric Final Rule. It emphasizes the need for pediatric studies for all new drugs, addressing critical factors such as dosage, route of administration, and therapeutic benefit while outlining compliance requirements and potential waivers. Additionally, it covers clinical considerations for drug safety evaluation, pharmacokinetics, and developmental toxicity in various pediatric age groups.