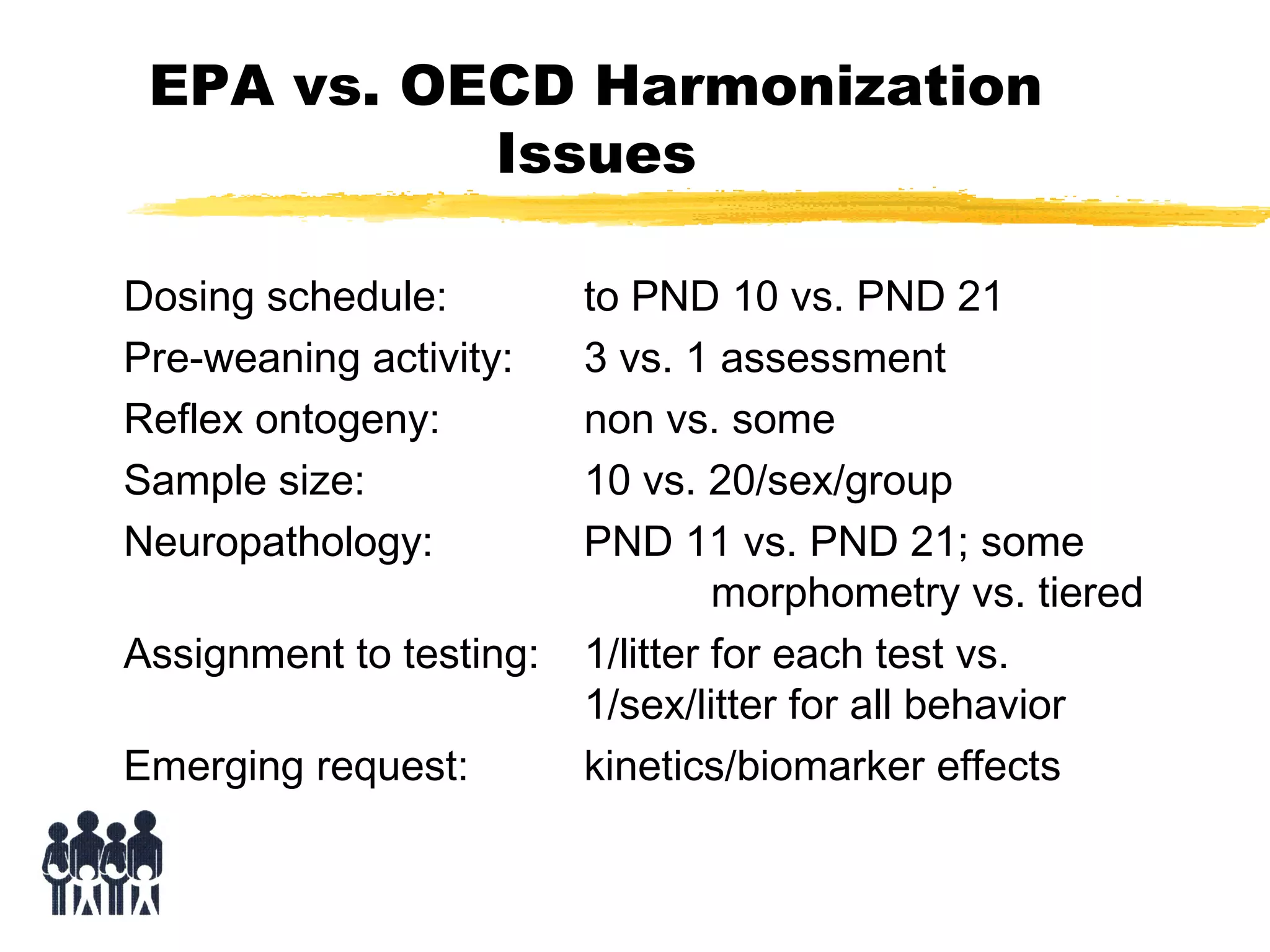
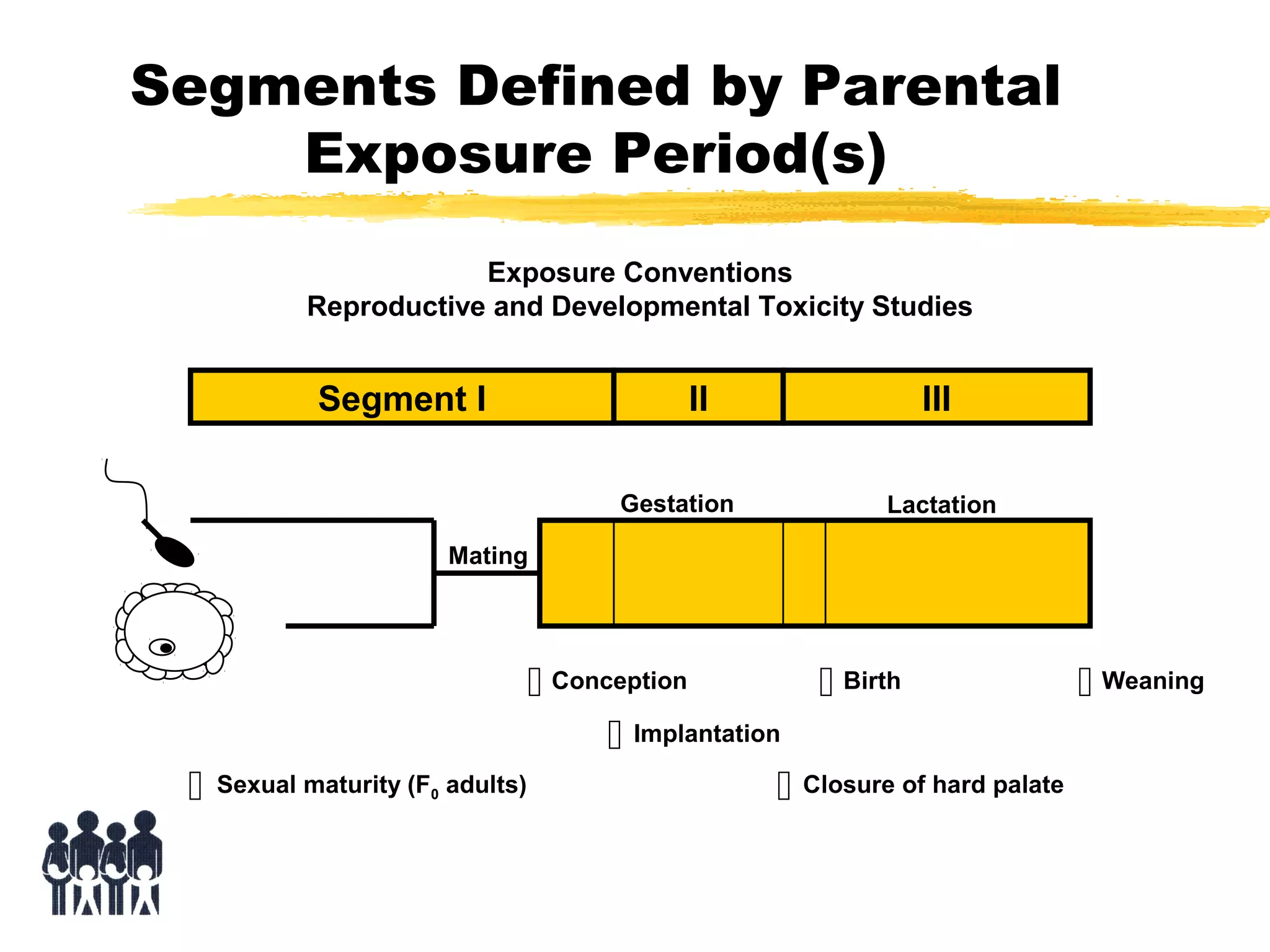
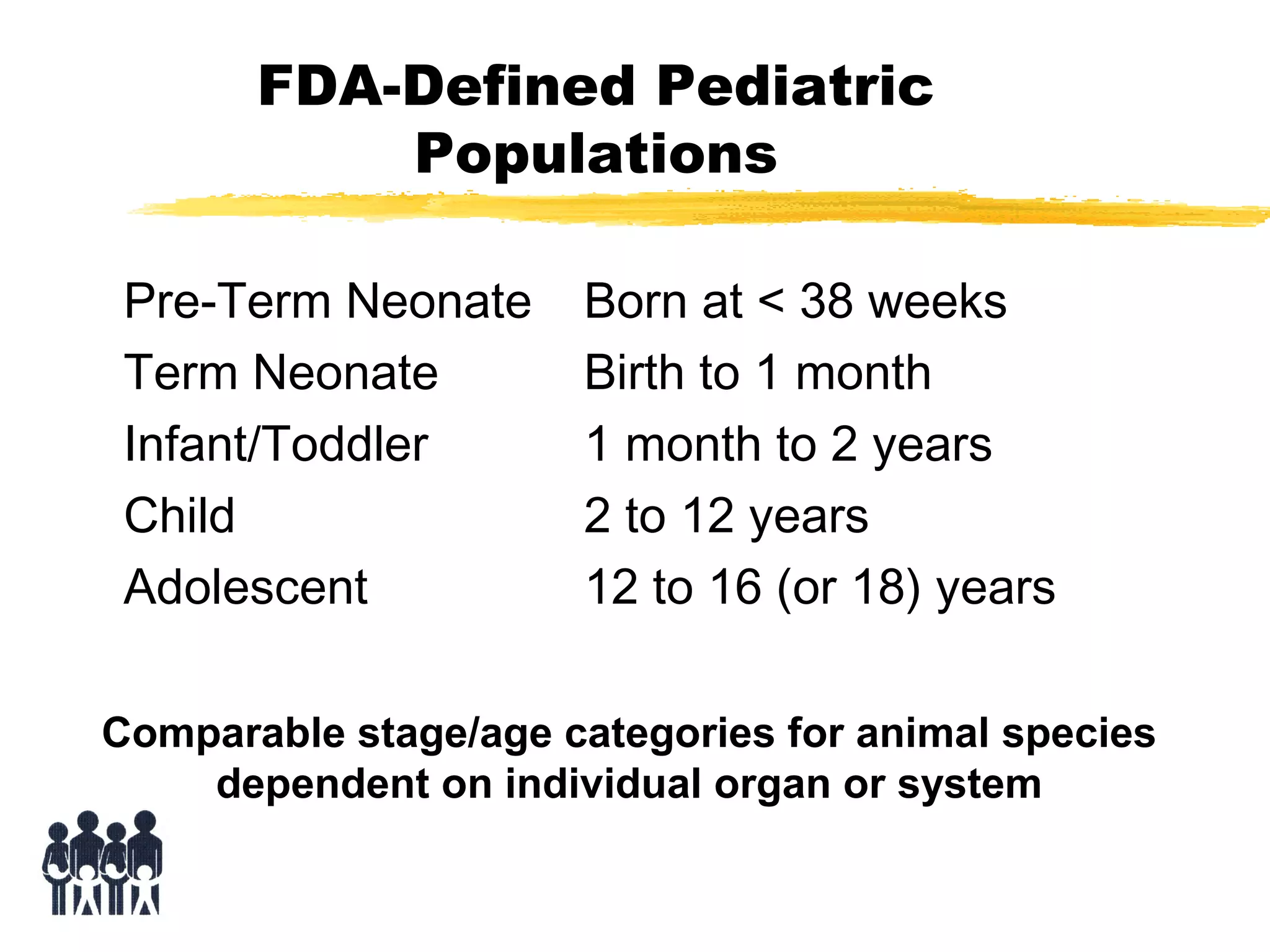
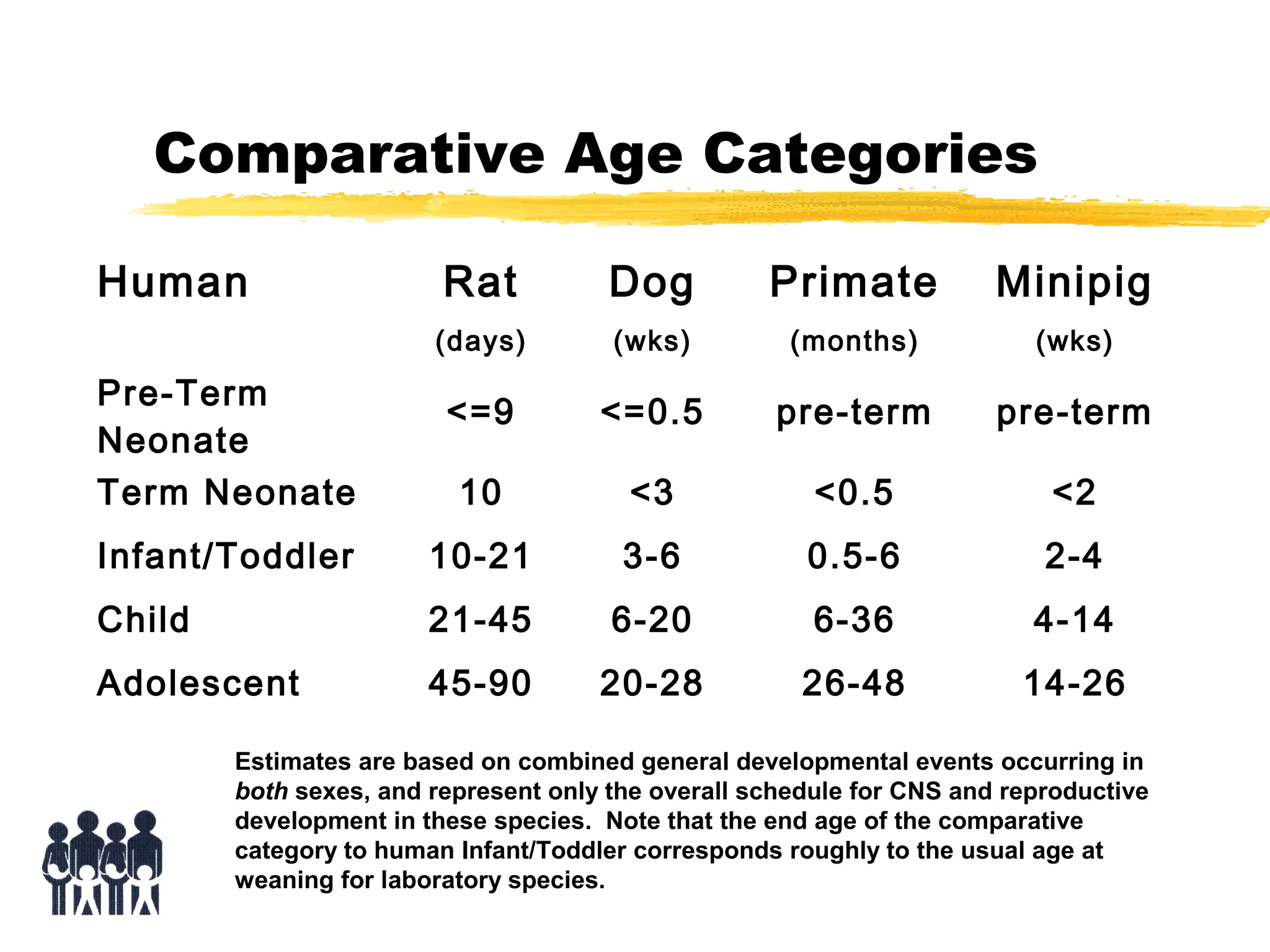
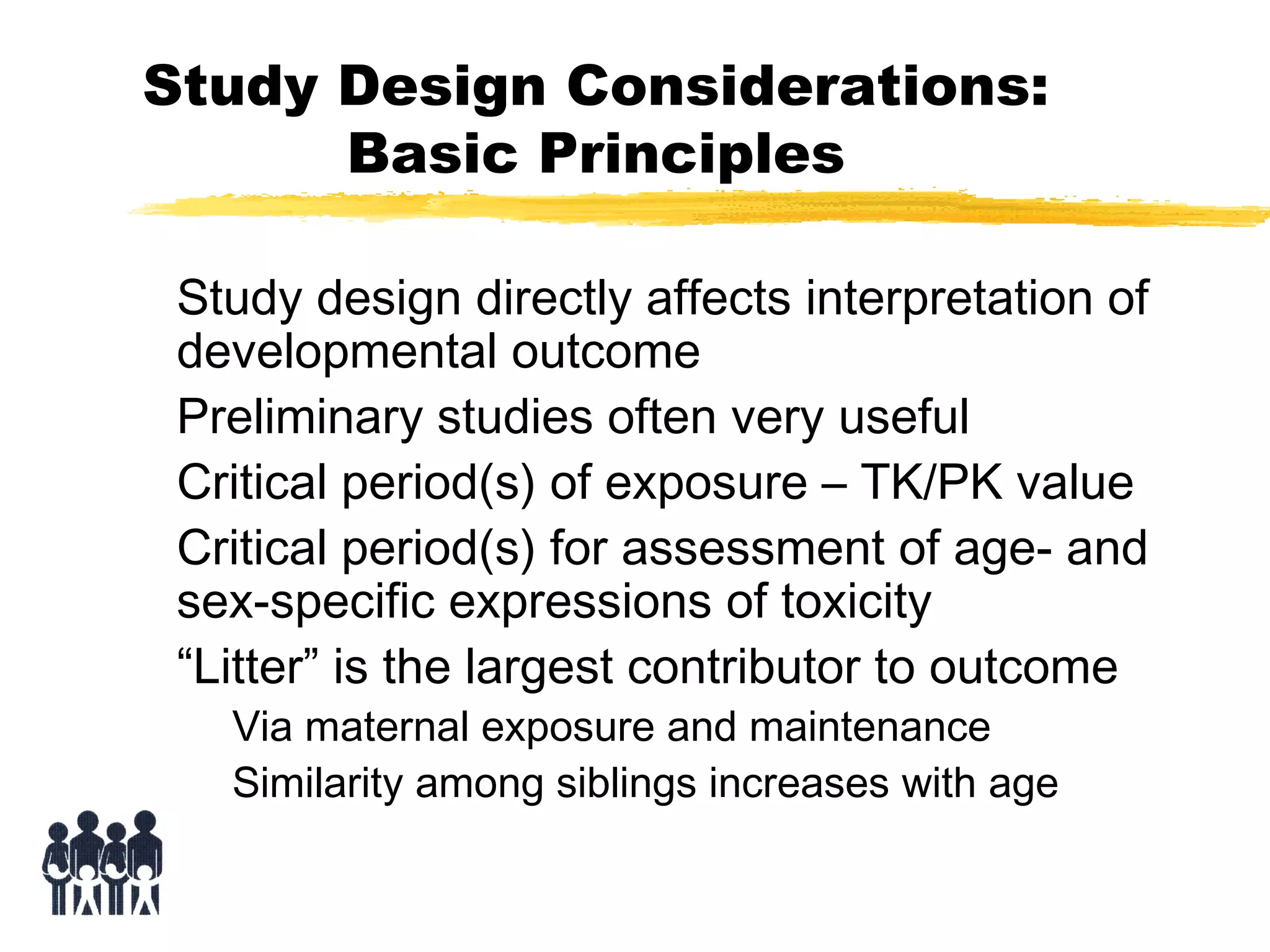
This document discusses guidelines for postnatal evaluation in developmental and juvenile toxicity studies. It compares EPA and OECD harmonization issues for dosing schedules, assessments, sample sizes, and neuropathology in two-generation reproduction and developmental neurotoxicity studies. The document also discusses guideline requirements for testing pharmaceuticals, including evaluating postnatal outcomes after parental exposure. It considers study design factors like target organ systems, exposure periods, basic principles, and whether direct neonatal exposures are needed.