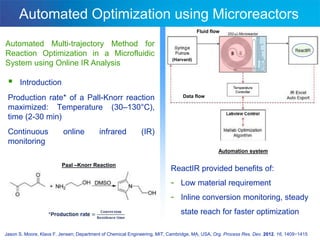
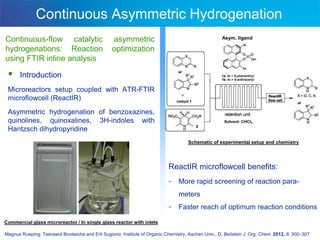
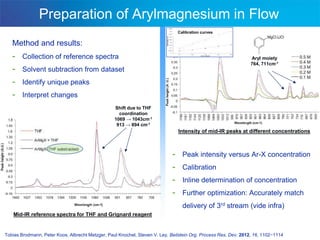
This document discusses several studies utilizing continuous flow microreactors for organic synthesis. One study produced an unstable Vilsmeier-Haack formylation intermediate in a safe and controlled manner using inline infrared analysis to optimize reaction conditions. Another used inline infrared to study gas-liquid homogeneous catalysis kinetics at high pressures. A third demonstrated automated optimization of a Pall-Knorr reaction using online infrared data in a microfluidic system.




![Flow Production of Unstable Intermediates
Formation of the VH-reagent
Plot [2] and [3] as a function of residence
time
Higher [3] level at Rt>100s possibly due
to higher [Cl-] resulting from counterion 2 3
degradation
Conclusions
IR 769 cm-1 VH formylation proved to be readily
IR 804 cm-1 conducted in flow microreactor system
2 FlowIR essential to solve at-line UV
limitations
3 Optimization of reaction time (180 s),
temperature (60 °C, molar ratio (1.5 eq.)
→ 5.98 g/h
A. M. W. van den Broek, J. R. Leliveld, R. Becker, M. M. E. Delville, P. J. Nieuwland, Kaspar Koch, F. P. J. T. Rutjes; FutureChemistry Holding BV,
Institute for Molecules and Materials, Radboud University Nijmegen; The Netherlands; Organic Process Research and Development, 2012, 16, 5,
934-938](https://image.slidesharecdn.com/recentadvanceswebinarpart8dominiquehebraultv1-130412082840-phpapp02/85/Recent-Advances-Webinar-Part-8-5-320.jpg)