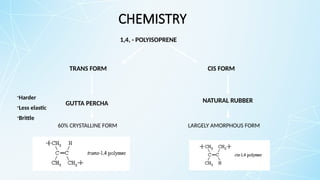
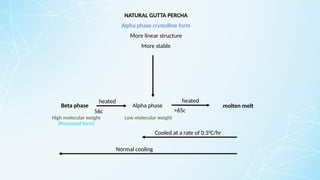
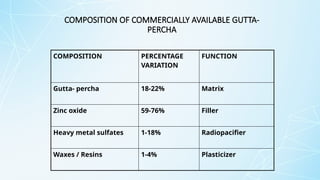
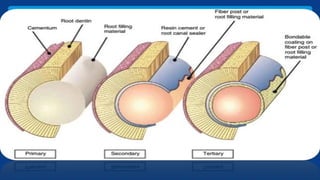
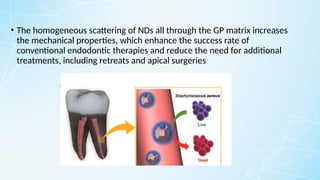
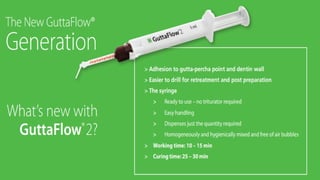
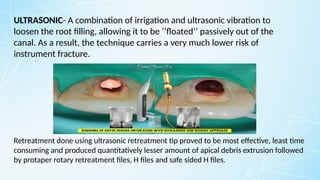
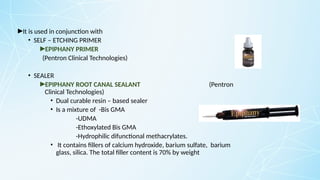
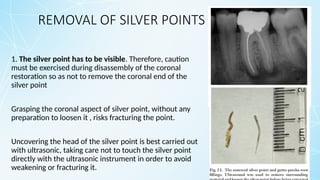
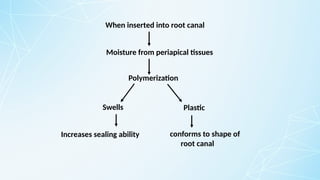
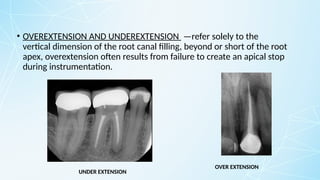
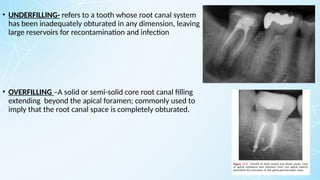
Obturating materials are an essential component of endodontic therapy, playing a critical role in the long-term success of root canal treatment. The primary goal of obturation is to completely fill and seal the cleaned and shaped root canal system to prevent reinfection by bacteria and their byproducts.














![CLASSIFICATION OF MATERIALS USED FOR
OBTURATION
• Solid core materials
1. Silver cones
2. Resilon
• Semi solid core materials
1. Gutta – percha
2. MGP [medicated gutta-percha points]
Eg : Gutta-percha with calcium hydroxide
According to Ingle](https://image.slidesharecdn.com/recentadvancesinobturatingmaterials-250406181228-4d171101/85/Recent-Advances-in-Obturating-materials-pptx-19-320.jpg)