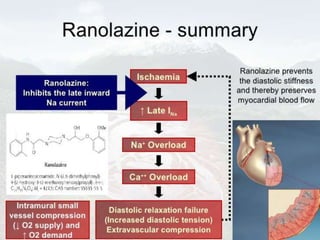
Ranolazine is a second-line antianginal drug that inhibits late inward sodium currents, reducing calcium overload and improving myocardial perfusion. It has shown efficacy in reducing angina in clinical trials. Ranolazine also partially inhibits fatty acid oxidation, shifting metabolism from fatty acids to glucose. Common side effects include dizziness and constipation. It has potential utility for various conditions including microvascular coronary disease and post-operative atrial fibrillation, though evidence is limited for others such as acute coronary syndrome.