Embed presentation


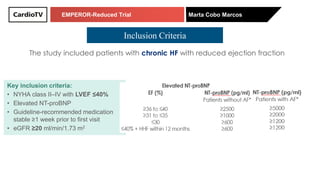
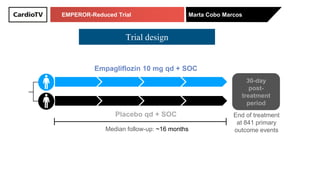

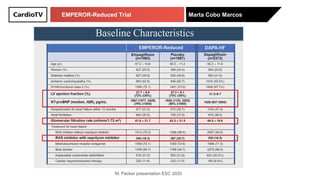
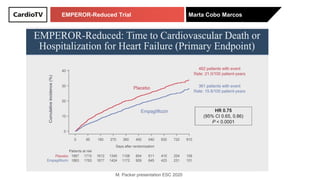
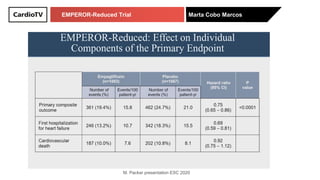
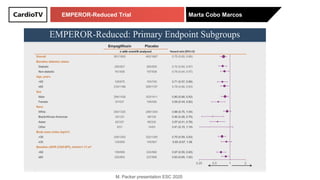

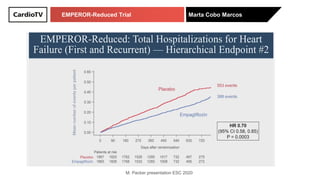
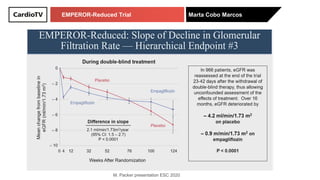
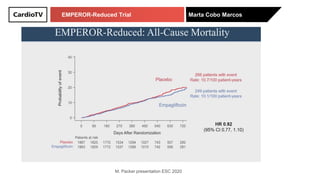
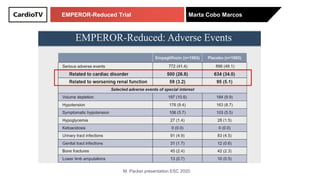


The EMPEROR-Reduced trial studied the effects of empagliflozin in patients with chronic heart failure with reduced ejection fraction. The trial included patients with NYHA class II-IV heart failure and an ejection fraction below 40% who were receiving standard guideline-directed medical therapy. Patients were randomly assigned to receive empagliflozin 10 mg daily or placebo and followed for a median of 16 months. The study found that empagliflozin reduced the risk of the composite primary outcome of cardiovascular death or hospitalization for heart failure compared to placebo.














