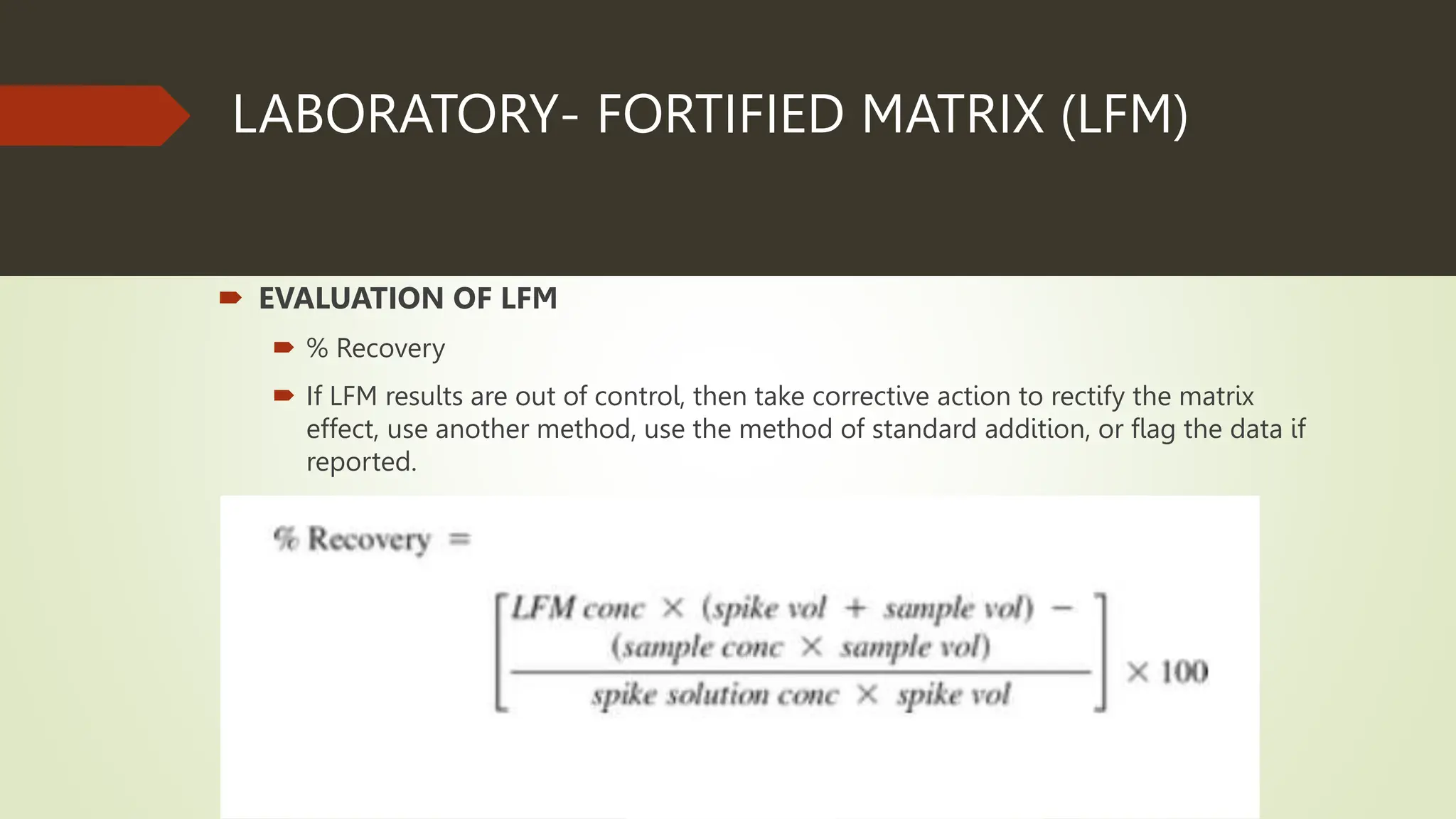
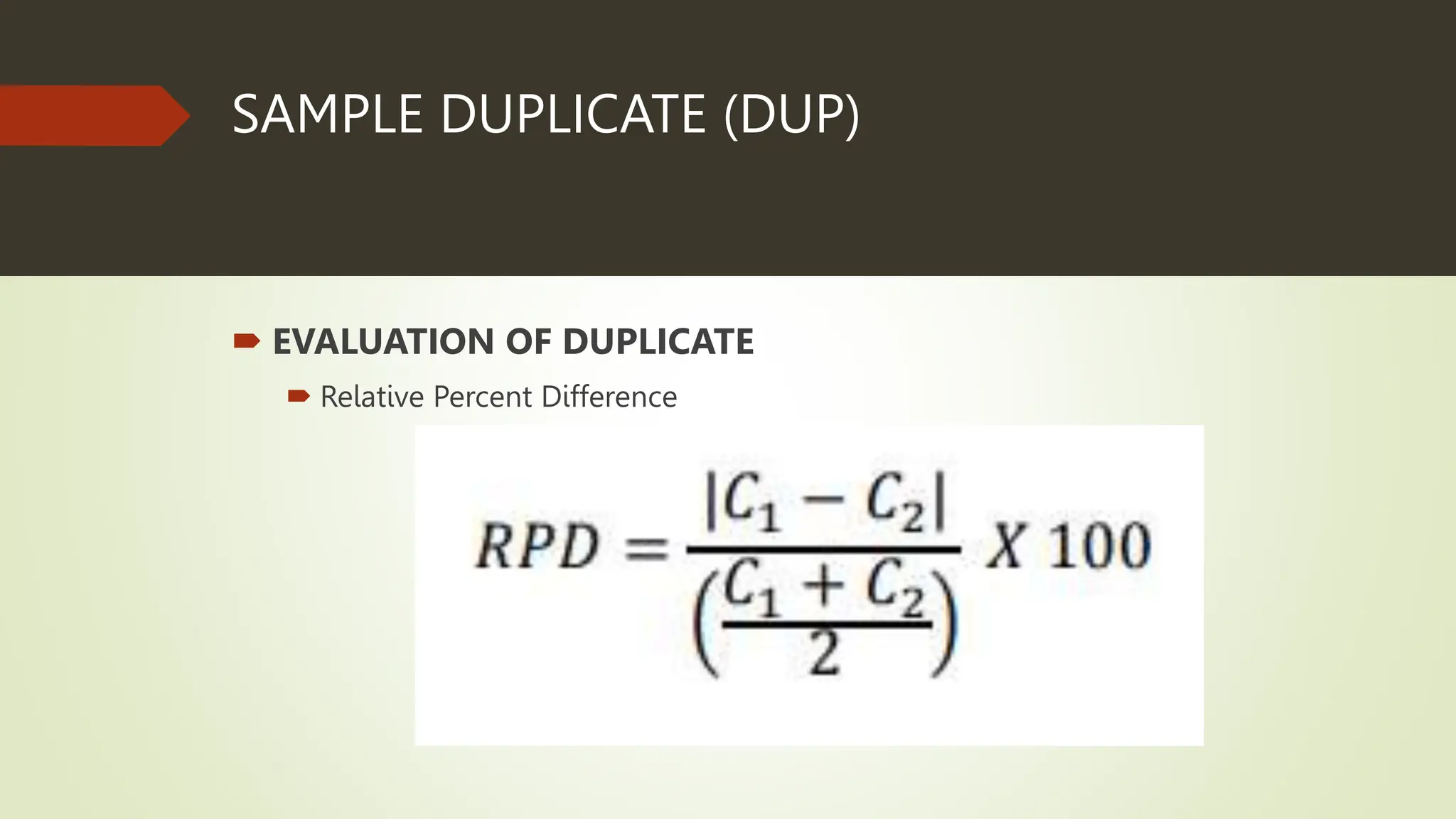
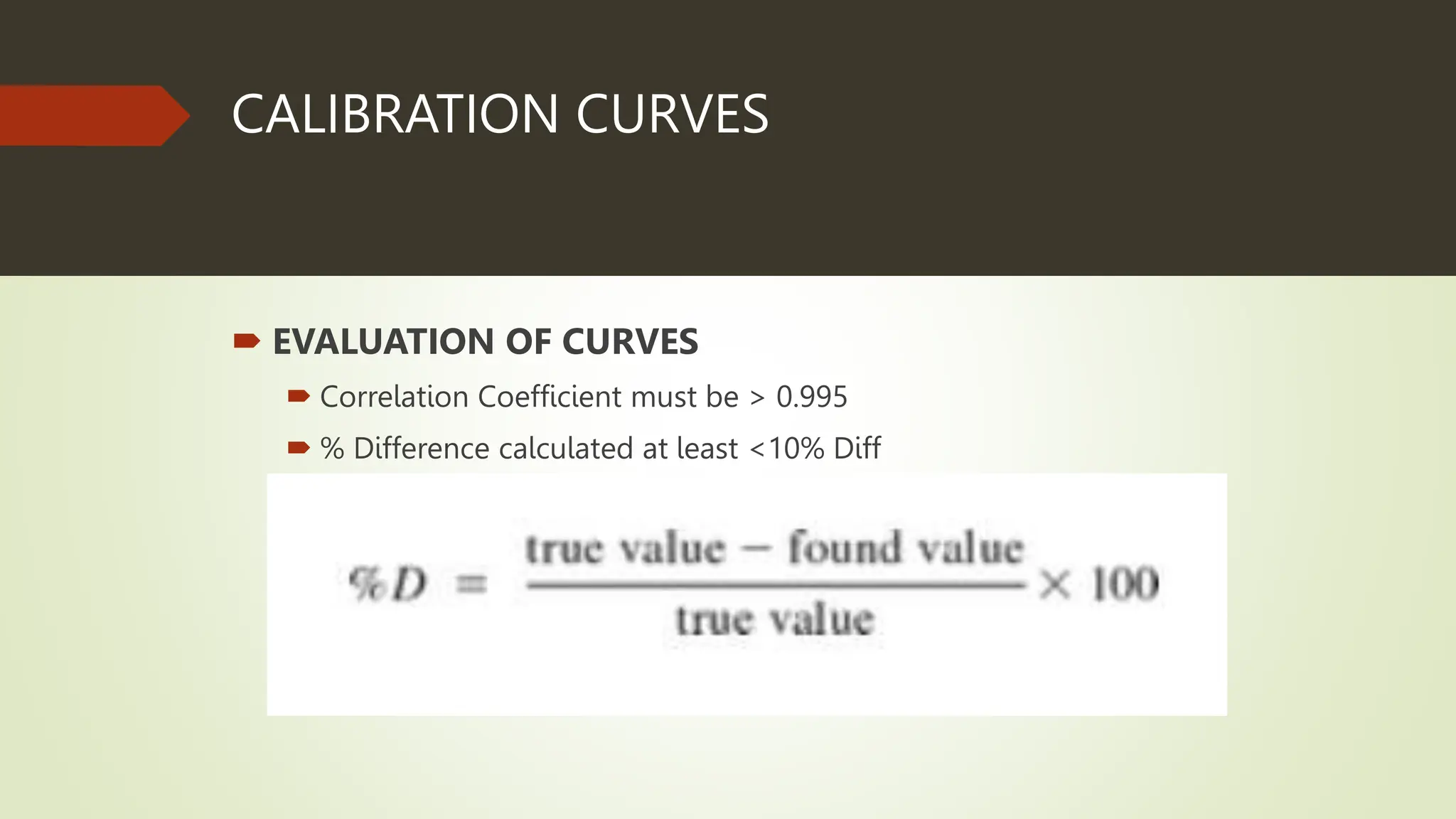
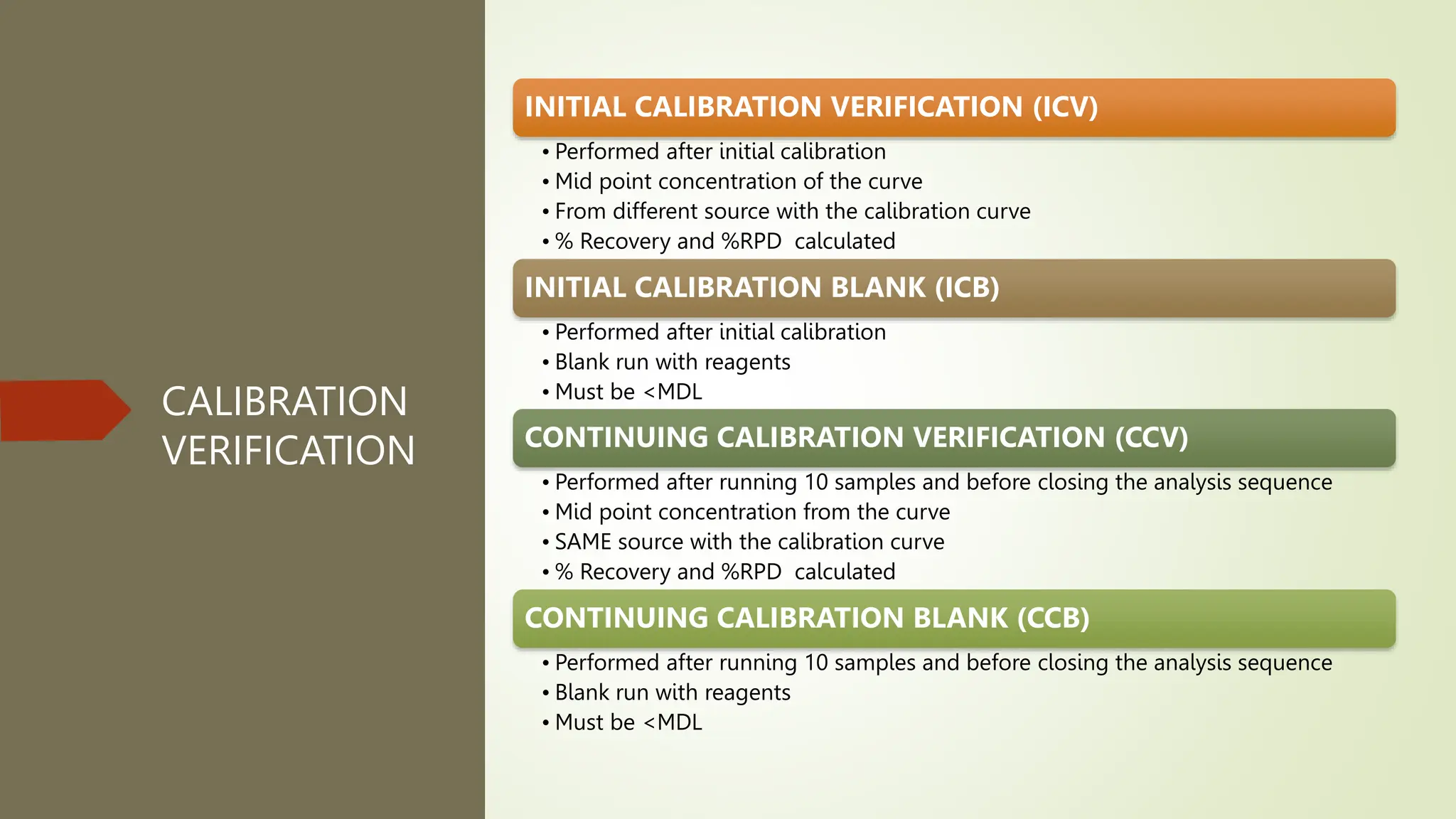
Each analytical batch contains quality control samples such as method blank, laboratory control sample/duplicate, sample duplicate and sample/duplicate which are analyzed according to standard operating procedures. The method blank is used to document contamination and samples are qualified if blanks exceed thresholds. Laboratory control samples assess accuracy and are evaluated using percent recovery and control charts. Sample duplicates evaluate precision using relative percent difference.