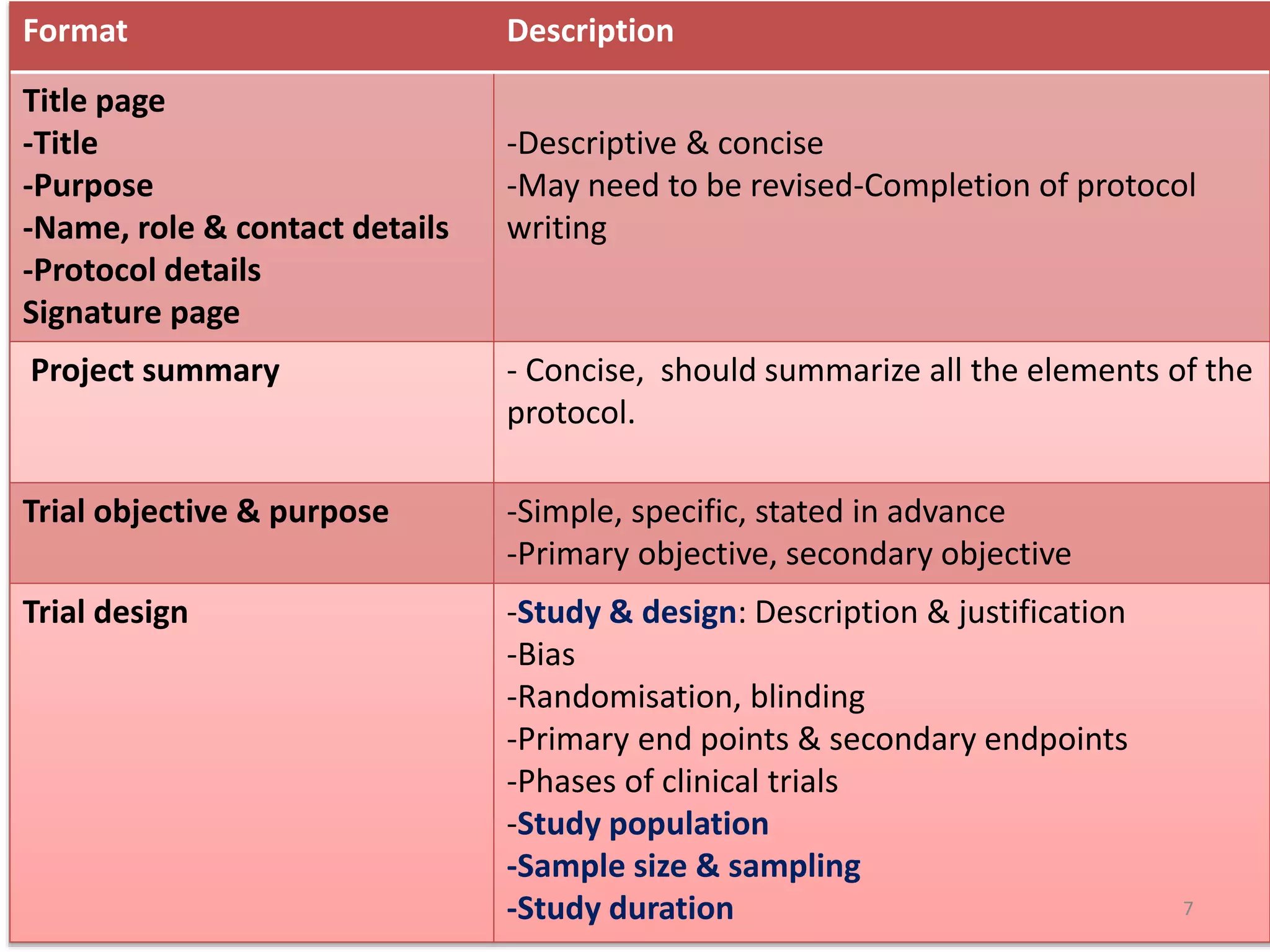
This document provides information on writing research protocols. It defines a research protocol as a study plan that describes the objectives, methodology, participants, procedures, and assessment tools for a clinical trial. The document then outlines the typical format for a research protocol, which includes sections for the title, summary, objectives, design, participant selection and withdrawal, treatments, efficacy and safety assessments, statistics, quality control, ethics, data handling, budget, publications policy, and references. It emphasizes that the protocol should provide a concise but detailed description of all aspects of the planned study.