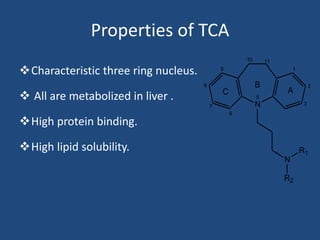
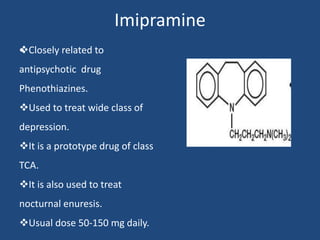
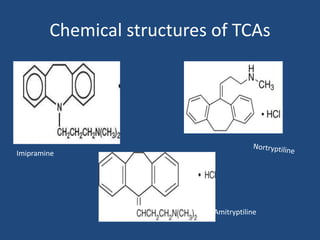
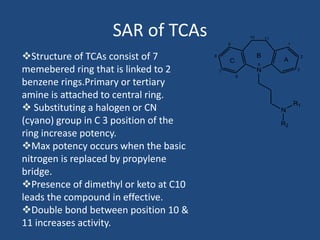
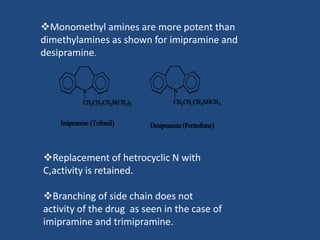
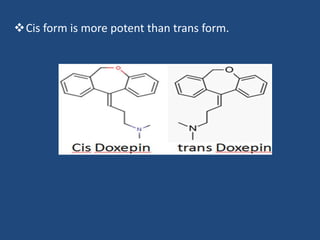
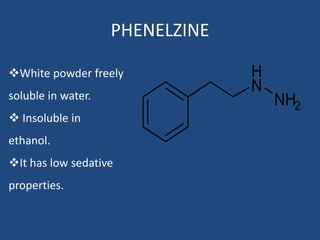
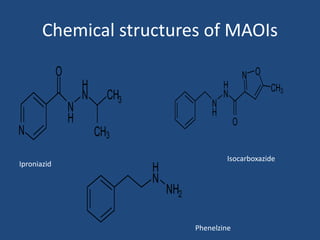
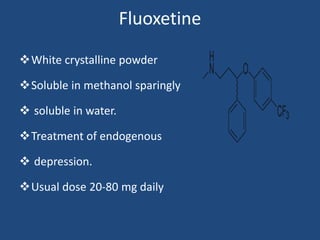
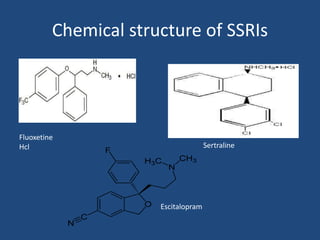
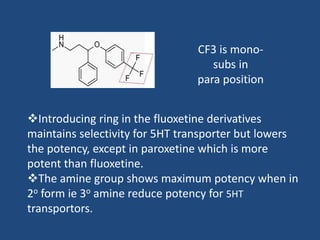
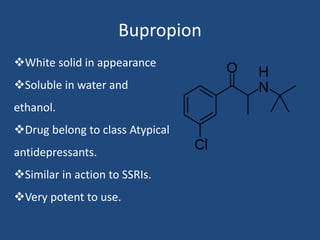
Depression is a treatable mental illness characterized by changes in mood and loss of interest. Antidepressants work by increasing levels of neurotransmitters like serotonin, dopamine, and norepinephrine in the brain. There are several classes of antidepressants including tricyclic antidepressants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, and atypical antidepressants. Each class has different mechanisms of action, side effects, and prescribing considerations. Antidepressants generally take 4-6 weeks to take effect and should be tapered gradually rather than stopped abruptly.